ESCAPING FROM PREDATORS An Integrative View of Escape Decisions (2015)
Part III Related behaviors and other factors influencing escape
13 The physiology of escape
Yoav Litvin, D. Caroline Blanchard, and Robert J. Blanchard
13.1 Introduction
In this chapter we review physiological mechanisms and correlates of flight or escape behavior. Although aspects of the physiology of flight have been studied in many prey taxa, our knowledge of physiological mechanisms has largely been obtained in studies of rodents. In much of this book, the focus is on decisions to flee, especially the decision about how close to allow a predator to approach before beginning to flee. In a typical field study, this is measured as flight initiation distance (FID), the distance between an approaching predator and the prey when the prey starts to flee. In part because the vast majority of studies of the physiology of escape have been done in laboratories in small spaces, very little is known about physiological influences on FID. However, flight is one component of a complex array of defensive behaviors to predators and other threat stimuli that has been intensively investigated in recent years, and a substantial body of information has accumulated about the endocrine, pharmacological, and neuroanatomical systems involved in defense. These findings are outlined here.
13.2 Defensive behaviors and physiology
13.2.1 Defensive behaviors (see Figure 13.1 for summary)
Defensive behaviors comprise a group of immediate and direct behavioral reactions to threats to life and bodily safety (Blanchard et al. 2009). Many defensive behaviors are evolved responses to the types of stimuli and situations that were frequent dangers in the evolutionary histories of a species, evolving as a result of the survival/reproductive success that they afforded to individuals displaying them appropriately. In this context “appropriately” means not only that the defenses be well executed, but also that each individual defensive behavior is one that has been particularly successful in response to that particular type of threat, and in that particular type of situation. Flight, avoidance, freezing, defensive threat, defensive attack, and risk assessment to threatening stimuli have been characterized in a variety of species, as have some other behaviors, e.g., burying of novel, aversive, or potentially dangerous objects, or alarm cries, that may be functional in particular threat situations (Blanchard 1997; Litvin et al. 2008).
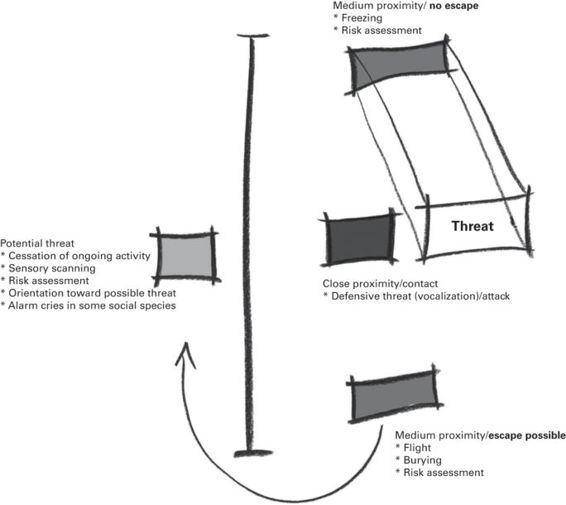
Figure 13.1
Fear- and anxiety-like defensive behaviors are modulated by subject-threat distance and context. Notably, the availability of escape enables flight, while its absence promotes freezing.
(Used with permission from Litvin & Pfaff 2013)
13.2.2 Endocrine roles in defense
Evolution has shaped organisms that are adequately adapted to a dynamic, at times hostile, environment. The brain detects a stimulus from the environment, integrates it with internal states, and in response potentiates appropriate physiology and behavior. The process of maintaining a constant, stabile internal milieu in a changing setting is termed “allostasis” and the ability to continually adapt by learning appropriate behaviors indicates “resilience” (Karatsoreos & McEwen 2013). Chronic stress leads to wear and tear on the body and brain and is thus termed “allostatic load” (McEwen 2007). In situations when an organism is faced with a threat, correctly executed escape behaviors are essential for survival and future propagation of the species. Further, after an escape is successful a quick return to non-defensive behaviors, e.g., territorial defense, foraging, or copulation, promotes rank within a social hierarchy and reproductive success (Blanchard & Blanchard 1989).
W. B. Cannon and P. Bard were the first to systematically examine the physiology of escape in the first half of the twentieth century (Cannon 1915, 1927; Bard 1928). Cannon and Bard’s findings linked activity in the brain stem and hypothalamus with behavioral and peripheral manifestations of emotions. Their studies provided the first associations between secretion of epinephrine from the adrenal medulla, peripheral mobilization, and emotional responses, indicating that the adrenals play a role in activation of the muscles and promote sugar metabolism. This “fight-or-flight” response facilitates increases in heart rate, circulation rate and depth, rate of respiration to facilitate oxygenation of necessary muscles, sweating for temperature regulation, increases in glucose metabolism for energy, redirection of blood from the skin and gut to muscles, and increases in blood clotting in preparation for bodily injury. In addition, the sympathetic branch of the autonomic nervous system facilitates the cognitive enhancement due to epinephrine release from the adrenal medulla.
Autonomic activation begins at the central nucleus of the amygdala, wherein corticotropin-releasing factor (CRF) is synthesized and subsequently released into the locus coeruleus, stimulating the release of norepinephrine and epinephrine into the general circulation, which in turn activate the peripheral autonomic response including cardiovascular responses (heart rate and blood pressure), body temperature regulation, perspiration, and bronchiole dilation (Cannon 1915, 1927).
The endocrine stress response also involves activation of the hypothalamic-pituitary-adrenal (HPA) axis. This process commences when neuroendocrine cells in the medial parvocellular division of the paraventricular nucleus of the hypothalamus (PVN) release the 41 amino acid neuropeptide CRF (Spiess et al. 1981; Vale et al. 1981) as well as oxytocin and arginine vasopressin (Turner et al. 1951; Tuppy 1953; Du Vigneaud et al. 1953). CRF travels axonally to the median eminence and is subsequently released into the hypophysial portal system, which leads to the anterior pituitary. At this location it binds to corticotrophs to stimulate the release of adrenocorticotropin hormone (ACTH). As a result, ACTH is released into the bloodstream where it causes the secretion of glucocorticoids from the adrenal cortex: cortisol in humans and corticosterone (CORT) in rodents. The HPA axis is tightly controlled by a number of central negative feedback sites; specifically, the hypothalamus, pituitary, and hippocampus have been identified as major brain targets of glucocorticoid-mediated regulation (see Herman et al. 2005 for a review). Glucocorticoids mobilize energy by promoting catabolism of proteins, glycogen, and triglycerides, stimulating gluconeogenesis in the liver and kidneys, and augmenting cardiovascular effects of catecholamines at target tissues by enhancing epinephrine release and sharpening cognition. Glucocorticoids also inhibit physiological and biochemical processes in the body that are either unnecessary in times of danger or can interfere with appropriate escape. These include suppression of systems related to digestion, reproduction, and immunity. Although both CRF and glucocorticoids aid in the facilitation of an appropriate response to stressors that ultimately enhances chances of survival, chronic release of these hormones has been shown to produce deleterious effects on a number of bodily processes, such as immunity, digestion, learning and memory, and reproduction (McEwen 2007).
The level of HPA activation, i.e., glucocorticoid release, is used as a valid measure of the health of an individual and of a population within an ecological system (Romero 2004). As such it is important to notice the factors that affect glucocorticoid release in response to a stressor when examining physiology of defensive behaviors such as flight. These include genetic influences on the HPA axis as well as epigenetic factors. Genetic factors determine glucocorticoid receptor distribution, which in turn affect rate of termination/recovery from HPA activation, while epigenetic factors include early-life experience (trauma/enrichment), subjugation to chronic stressors during the lifetime of the animal, all of which can alter expression of genes relevant to a stress response (McEwen 2007).
Stress produces structural remodeling in the hippocampus, amygdala, and prefrontal cortex with these changes altering behavioral and physiological responses to subsequent stressors (McEwen 2007). For example, stress-induced activation of adrenergic and glucocorticoid receptors modulates memory-enhancing effects that facilitate resilience by regulating defensive behavior (escape, among others) toward a learned stimulus (Lupien & McEwen 1997; McGaugh & Roozendaal 2002; Karatsoreos & McEwen 2013). Glucocorticoids are also involved in neuronal excitability and long-term potentiation in the hippocampus, consistent with a role in memory storage (Diamond et al. 1992; Joels & de Kloet 1992). The hippocampus is a major target of adrenal steroids and is said to modulate memory processes via intracellular cascades that result from glucocorticoid and adrenergic receptor activation. The putative molecular mechanisms involve glucocorticoid-mediated expression of neural cell adhesion molecules, which in turn cause synaptic structural changes (McGaugh & Roozendaal 2002). In fact, post-training injections of corticosterone, the principal rat glucocorticoid, or other specific glucocorticoid agonists, into the hippocampus enhance memory consolidation in an inverted-U fashion, with lesions of the basolateral nucleus of the amygdala blocking this type 2-glucocorticoid receptor (GR)-mediated effect (Micheau et al. 1984; Roozendaal et al. 1997, 2004).
A single study suggests that glucocorticoids affect flight initiation distance (FID) in the field. In the lizard Sceloporus undulatus, FID increases during a series of successive approaches by a predator (Thaker et al. 2010). However, following injection of metapyrone, which blocks synthesis of corticosterone, FID failed to increase during repeated approaches (Thaker et al. 2010). This study suggests that some of the physiological mechanisms influencing escape and related behaviors in laboratory tests may apply as well to FID and related behaviors in the field.
13.2.3 Anatomy/neurochemistry of flight-relevant defense systems
Several excellent reviews (e.g., Johnson et al. 1995; Carrasco & Van de Kar 2003; Quintino-dos-Santos et al. 2014) have documented the history of attempts to describe the brain circuitry involved in defensive behaviors. A major difficulty in relating these unequivocally to flight is that there may be quite different brain systems underlying defensiveness to different types of threat stimuli, even though most or all of these stimuli can elicit flight as a consistent and substantial component of the defense pattern. Canteras and Graeff (2014), provide information, and indeed schematics, of focal neuroanatomic components of the defense systems, for defensiveness to stimuli conditioned to painful experiences; and to fear based on predator exposure or on social defeat. In addition, Canteras and Graeff (2014) describe neural systems underlying fear to interoceptive challenges such as cardiac arrhythmias, visceral pain, and hypoxia, all of which represent threat to the organism. None of the latter is associated with a substantial range of potentially effective defensive behaviors - indeed no behavior is likely to remedy an acute cardiac arrhythmia - but it is notable that hypoxia, associated with suffocation, might be expected to involve behavioral attempts to deal with the cause of the suffocation, potentially including fight or flight responses (Klein 1993). These different types of threat stimuli and how they may be differentially involved in activity in specific sites in the brain are discussed extensively in Gross and Canteras (2012).
Predator stimuli, to this date more often used than any natural threats in analyses of the neuroanatomy of defensiveness, typically involve a cat, or cat fur/skin odor, or odors derived from another predator, e.g., trimethylthiazoline (TMT), a component of fox feces (Apfelbach et al. 2005). These predator stimuli all elicit some elements of defense, but there are striking differences among the specific behaviors seen: cat exposure typically involves confrontation of the (usually rat) subject with a cat that is caged or otherwise restrained from actual contact with the subject. In most such situations, flight is limited by the dimensions of the test cage, and freezing is by far the predominant response seen: for example, Motta et al. (2009) reported that about 97% of a five-minute test period involving confrontation of a rat with a caged cat involved freezing. While it is plausible that the motivation to flee was definitely present for these rat subjects, flight itself, and the neural circuitry supporting the actual behaviors involved in flight, would be expected to be less activated in these tests. Similarly, cat odor tends to involve freezing, but this may be mixed with some approach - investigational activities (risk assessment) that are typically associated with more ambiguous threat stimuli (Blanchard et al. 2011). Trimethylthiazoline, while it does not robustly support fear learning (Blanchard et al. 2003b) and produces a brain activation pattern rather different than cat odor (Staples et al. 2008), is also used extensively for studies of the physiology of defense (Rosen 2004). Freezing rather than more active defense is the actual behavior associated with the brain activation in most of these studies. However, it is clear that confrontation with a predator does indeed elicit flight when this is possible, so these studies are nonetheless relevant to analyses of the neural systems involved in flight/escape. In keeping with the focus of this book, findings from the predator exposure model(s) will be the center of attention here.
Predator odors activate olfactory pathways projecting to the medial amygdala, and after predator (cat) odor exposure activation of the immediate early gene c-Fos is seen specifically in the posteroventral part of the medial amygdala (MeApv; Dielenberg et al. 2001), with additional high-level expression (compared to that seen with a strong but non-predator odor) in the dorsomedial part of the ventromedial nucleus of the hypothalamus (VMHdm), the hypothalamic dorsal premammillary nucleus (PMd), and the dorsomedial, dorsolateral (and more caudal levels), and the ventrolateral periaqueductal gray matter of the midbrain (PAGdm, PAGdl, and PAGvl, respectively). These findings are in agreement with reports from Canteras et al. (2002) of the organization of hypothalamic nuclei activated in exposure of a rat to a live cat (see Figure 13.2 for summary). Notably, these findings implicated the basolateral and lateral nuclei of the amygdala as well as the MeA. The former receive inputs from a number of sensory systems, likely reflecting additional sensory information about the cat, rather than the cat odor alone that was provided in Dielenberg et al. (2001).
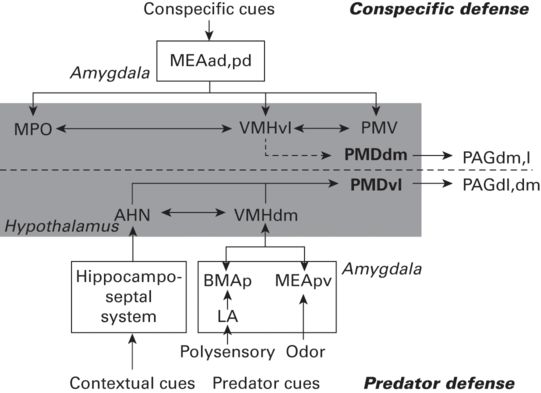
Figure 13.2
A schematic diagram showing the putative brain systems involved in processing predatory and conspecific threats and in organizing predator and conspecific defense. Abbreviations: AHN, anterior hypothalamic nucleus; BMAp, basomedial amygdalar nucleus, posterior part; LA, lateral amygdalar nucleus; MEAad, -pd, and -pv, medial amygdalar nucleus, anterodorsal, posterodorsal, and posteroventral parts; MPO, medial preoptic area; PAGdl, -dm, and -l, periaqueductal gray, dorsolateral, dorsomedial, and lateral parts; PMDdm and -vl, dorsal premammillary nucleus, dorsomedial, and ventrolateral parts; PMV, ventral premammillary nucleus; VMHdm and -vl, ventromedial nucleus, dorsomedial and ventrolateral parts.
(Used with permission from Motta et al. 2009)
With reference to this core set of hypothalamic nuclei, the medial amygdala, projects via the bed nucleus of the stria terminalis, to the VMHdm (Cezario et al. 2008). In addition, the MeA projects to the ventral hippocampus and in turn information from the hippocampus projects via the septum to the anterior hypothalamic nucleus (AHN), and the lateral hypothalamic area (LHA) (Petrovich et al. 2001). Lesions of the ventral hippocampus reduced freezing and enhanced non-defensive behaviors to cat presentation (Pentkowski et al. 2006). Strong involvement of the hippocampus in spatial representation (O’Keefe & Nadel 1978) suggests that these projections may carry information relative to the context in which the predator stimuli appear (a crucial feature in the choice of defensive behaviors, especially the balance between freezing and flight (Blanchard & Blanchard 2008). The latter areas are interconnected, and connect also to the VMHdm, and all three of these project to the PMD (Cezario et al. 2008), that, in turn, projects to dorsal parts of the PAG.
Cytotoxic lesions or muscimol (GABAA agonist-induced) blockade of the PMd produced profound reductions in defensive responding to cat-related stimuli and also a reduction in Fos activation of the dorsal PAG (Cesario et al.2008). These relationships are schematized in Cesario et al. (2008), including an additional connection of the PMd to the ventral part of the anteromedial nucleus of the thalamus. This area provides a connection to a component of the cingulate cortex that may be involved in eye/head movements associated with attention (Risold & Swanson 1995). Canteras et al. (2008) reported that the NMDA antagonist AP5, injected into the PMd, also reduced conditioning to a coffee-odor stimulus associated with footshock, consonant with a view that the PMd, through its connections to the cingulate cortex, may be accessing attentional and associational mechanisms for defensive behavior.
A number of recent studies have outlined differences in the neural circuitry of defensive behaviors elicited by different types of threat stimuli (Motta et al. 2009; Gross & Canteras 2012; Canteras & Graeff 2014), finding substantial differences for systems associated with responsivity to predators, conspecifics, and conditioned threat stimuli associated with pain. However, in contrast to its immediate responsivity to predator stimuli, defensiveness to conspecifics in laboratory rats tends to require contact, and the pain of biting attack (Blanchard & Blanchard 1989). A bitten animal quickly displays defensive behaviors that have been differentially evaluated, in one recent study of associated neural activation (Motta et al. 2009), as active responses (flight, boxing, and an upright defense of pushing off the attacker) vs. passive responses (freezing and lying on the back - the so-called “submissive” posture). The Motta et al. (2009) studies thus provide the possibility to differentiate passive and active defenses in terms of areas activated during these behaviors, and to determine behavior changes when relevant areas are inactivated. They report that while predator exposure produces Fos upregulation in the ventrolateral portions of the PMd, conspecific threat/attack results in enhanced Fos in the dorsomedial PMd (Motta et al. 2009). Lesions that affect the entire PMd strikingly disrupt defensive behaviors to a cat, but when conspecific threat is used such lesions reduce only passive defenses, with active defenses showing a non-significant but substantial increase.
While this pattern of results does indicate a differentiation of PMd inactivation effects on active vs. passive defenses, suggesting that the PMd may be less involved with the former than with the latter, an alternative explanation is that it is consonant with the interpretation of a general decrease in the intensity of defensiveness after PMd lesions. In particular, lying on the back or “submission” is a high-intensity response to threat, with accompanying signs of autonomic and glucocorticoid arousal (e.g., Fokkema & Koolhaas 1985; Huhman et al. 1990), whereas active defenses such as upright pushing away of the opponent and flight may under some circumstances actually reduce stress responsivity (Viken et al. 1989). Thus a reduction of “passive” in favor of more active defensive responses may reflect a change in the intensity of the threat motivation rather than alteration of a system underlying a specific type of defensive behavior.
13.2.4 Systemic drug effects on defensive behaviors, including flight
Preclinical animal models are utilized in the study of unconditioned and conditioned behaviors potentially related to fear and anxiety, with a view to understanding their neural and endocrine correlates, and their underlying etiology, and to screen novel pharmaceuticals aimed at alleviating these conditions. Such assays include high-throughput models; for example, the elevated plus-maze (EPM), open field, elevated T-maze (ETM), light/dark box, social interaction test, and separation-induced ultrasonic vocalizations. They also include more ethologically relevant models such as seminatural visible burrow systems (VBS) and other situations where rats or mice are confronted by predators or predator-related stimuli (Blanchard & Blanchard 1989; Litvin et al. 2008; Motta et al. 2009).
Two animal models: the elevated T-maze (ETM) and the mouse defense test battery (MDTB) are particularly relevant to flight/escape behaviors. The MDTB was created specifically to enable evaluation of the magnitude of a range of defensive behaviors of mice (Mus musculus) to a hand-held, anesthetized rat. Because rats are predators of mice (Blanchard et al. 2003a), they elicit strong defensive behaviors in mice, with the form or type of defense varying with the movements of the rat and features of the threat situation (Blanchard et al. 2003a). In the MDTB, in which features of the threat stimulus and situation are varied in order to elicit a number of different defensive behaviors in succession, profiles of changes in various defensive responses to a given drug manipulation can be obtained in a single test session.
Results of pharmacological manipulations of mice assessed using the MDTB suggest that a number of neuroactive chemicals may enhance or reduce defensive behaviors in a relatively non-specific fashion. Intracerebroventricular administration of CRF tends to enhance the predominant defensive behavior elicited by each stimulus combination of threat and situation utilized in the MDTB (Yang et al. 2006). Blanchard et al. (2001) reported that a number of benzodiazepines (BZPs) alter flight in the MDTB. However, for most BZPs there was no selective effect on flight with doses that did not impair motor function. However, two benzodiazepines, alprazolam given on a chronic, but not acute, basis (Griebel et al. 1995b) and clonazepam (Griebel et al. 1996) selectively impaired mouse escape responses. In addition, chronic administration of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine both robustly reduced flight (Griebel et al. 1995a; Beijamini & Andreatini 2003). In contrast, cocaine and yohimbine substantially and selectively increase flight compared to other aspects of defensiveness (Blanchard et al. 1993, 1999). As the drugs selectively reducing flight are clinically effective against panic, while cocaine and yohimbine may precipitate or enhance panic (Cox et al. 1990; Bourin et al. 1998), these findings suggest that flight may serve as a relatively selective animal model for panic disorder (Blanchard et al. 1993; Griebel et al. 1996).
The elevated T-maze (ETM) has been used even more extensively in the analysis of flight/escape responses. The ETM consists of three elevated arms - one enclosed and two open, forming a “T”. To assess inhibitory avoidance, a subject is placed at the end of the enclosed arm and the latency to exit this arm is recorded in three consecutive trials. Inhibitory avoidance learning is indicated by the increase in withdrawal latency across trials. Thirty seconds after the completion of avoidance training, the second behavioral task (one-way escape) is measured. For this, the animal is placed at the end of one of the open arms of the maze and the withdrawal latency from this arm is similarly registered in three consecutive trials.
The ETM was designed specifically to separate these defensive behaviors (Deakin & Graeff 1991) with the underlying premise that inhibitory avoidance and escape responses are differentially regulated by serotonin (5-HT) released from fibers in the dorsal raphe nucleus (DRN), a midbrain structure that innervates neural substrates particularly involved in defensive behaviors (e.g., amygdala, frontal cortex, dorsal periaqueductal gray (dPAG), among others) (Graeff et al. 1993; Viana et al. 1994). This emphasis on the separation of inhibitory avoidance of the open arms of the ETM and escape from the same open arms was associated with interpretation of the former as a model of generalized anxiety, while the latter, escape, was viewed as an animal model for panic. Zangrossi and Graeff (2014) have conducted a number of experiments to behaviorally validate the ETM. Their results showed that rats trained on an ETM with three enclosed arms did not show the increase in withdrawal latency along three consecutive trials that is often observed in the standard ETM procedure. Therefore, open arm experience seems to be critical for inhibitory avoidance learning. Moreover, over repeated trials in an open arm, animals left at increasingly higher speeds. Both findings are consonant with a view that the open arms are aversive, and that repeated placement in the closed arm or an open arm does elicit avoidance, or escape, respectively. Indeed, repeated open arm experience appears to enhance the sensitivity of the resulting escape response to drug effects.
Zangrossi and Graeff (2014) also summarize the effects of systemic drugs on escape from the open arms of the ETM. Briefly, reduction of escape from the open arms was produced by acute administration of the benzodiazepine alprazolam, and the SSRI paroxetine, with no effect from acute treatment of a number of additional benzodiazepines and 5-HT-acting drugs. Similarly, chronic treatment (9-14 days) with the benzodiazepine diazepam, and the 5-HT1A receptor partial agonist buspirone failed to alter escape. However, chronic treatment with a number of SSRIs, including sertraline, paroxetine, escitalopram, fluoxetine, and clomipramine all reduced escape, as did chronic treatment with the tricyclic imipramine. Moreover, most of these positive effects on escape were obtained in tests in which there was no alteration of the inhibitory avoidance effect, indicting a relatively selective effect of these treatments. Finally, as SSRIs are frequently the first choice drugs for treatment of panic, with effects that usually appear after chronic administration, while alprazolam is the only benzodiazepine with antipanic efficacy at non-sedative doses, this pattern of results is very supportive of a view that escape measured in the ETM can serve as an animal model of panic (Mochcovitch & Nardi 2010).
Additional evidence of involvement of serotonin in flight-escape is suggested by findings that injection into the dorsomedial hypothalamic nucleus (DMH) of the preferential 5-HT2a agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI), or of the 5-HT1a receptor agonist 8-OH-DPAT, raised the threshold for escape responses (running or jumping reactions) elicited by electrical stimulation of the DMH. Both of these effects were enhanced by chronic systemic administration of imipramine, a strong inhibitor of serotonin reuptake that also affects several other neurotransmitters, and the DOI effect was enhanced by chronic fluoxetine, which inhibits serotonin reuptake (de Bortoli et al.2006). As noted earlier, the relationship between these findings and antipredator flight is complicated, in that the DMH is not part of what is currently regarded as the antipredator circuitry of the hypothalamus, although it is prominently featured in neural responsivity to conspecific threat (Canteras & Graeff 2014). However, this difference in circuitry may be at least partly the result of a methodological difference between the use of predators and conspecifics as threat stimuli, with the latter, alone, being allowed to actually attack (and cause pain) to the subject in such experiments: this possibility requires additional investigation.
The same caveat, that the relevant locale may not be part of the antipredator circuitry of the brain, applies to orexins/hypocretins, which are hypothalamic peptides located preferentially in the perifornical area, that may also affect escape responses. Orexin is co-localized with glutamate and is involved in regulation of a number of neurovegetative activities (see Johnson et al. 2012 for review). Afferent connections to orexin neurons originate in a host of sites such as the septohippocampal system (Hahn & Swanson 2012) and involve neurochemicals such as GABA and serotonin (Johnson et al. 2012) that are important for defensiveness. In turn, orexin neurons project to a variety of putative defense areas. One of these, the dorsal raphe (Lowry et al. 2005), is of particular interest (see below).
Glutamate receptors, including the n-methyl-d-aspartic acid (NMDA) type, within the dorsal or dorsolateral PAG play a major role in the initiation of PAG-evoked defensive behaviors, specifically escape. Microinjection of NMDA into this area produced flight-related behaviors (galloping, jumping) in a dose-dependent manner, thus mimicking the response to electrical stimulation in this region (Bittencourt et al. 2004). In fact, NMDA-induced excitation of the dorsal PAG elicits a range of defensive behaviors, from freezing to escape, with freezing evoked at lower doses and escape, at higher levels (Cardoso et al. 1994).
The dorsal PAG expresses high levels of GABA (Ferreira-Netto et al. 2005). GABAergic fibers from the substantia nigra and the pars reticulata exert an inhibitory effect on aversive behavior induced by dorsolateral PAG stimulation (Coimbra & Brandao 1993). These GABA receptors are modulated in a dose-dependent manner by benzodiazepines (Bovier et al. 1982). Further evidence for GABA involvement in this region is that microinjection of GABA antagonists, such as bicuculline, into the midbrain tectum induce flight and autonomic reactions similar to those of the defense response (Brandao et al. 1982; Melo et al. 1992). As with NMDA agonists, escalating doses of GABA antagonists produce freezing and escape, respectively (Graeff 1990). In addition, prior treatment with chlordiazepoxide (a benzodiazepine hypnotic and muscle relaxant) inhibits escape induced by bicuculline, an antagonist of GABA receptors (Borelli et al. 2005) and prior bicuculline injection abolishes the inhibitory effect of benzodiazepines (Brandao et al. 1982). Collectively, these findings implicate a GABA-benzodiazepine system in the midbrain tectum as an inhibitor of escape.
The dorsal raphe nuclei of the midbrain receive input from orexin neurons in the hypothalamic perifornical area and provide an inhibitory serotonergic input to the dorsal PAG (Lowry et al. 2005). Drug injections into the dorsal raphe that enhance serotonin release from the PAG tend to inhibit flight in the ETM (Graeff 1997). Roncon et al. (2013) demonstrated an opioid effect in this area: morphine in the dPAG increased escape latency in the ETM. Moreover, both the 5-HT1a agonist 8-OH-DPAT and the 5-HT2a agonist DOI produced anti-escape effects, but only the first of these was antagonized by naloxone. Additional studies in this series (Roncon et al. 2013) supported an interaction of serotonergic and opioidergic mechanisms in the dPAG.
The SSRIs fluoxetine and paroxetine (as well as alprazolam) reduced flight-like escape behavior produced in rats by electrical stimulation of the dPAG (Hogg et al. 2006). These data are in agreement with ETM findings for dPAG injections of the same agonists, again in rats (Zanoveli et al. 2003; de Paula Soares & Zangrossi 2004). Connecting this escape measure to the flight seen in the MDTB and providing considerable evidence of consistency across rat and mouse subjects, injections of the 5-HT1A agonist 8-OH-DPAT, and the preferential 5-HT2A receptor agonist DOI into the dPAG, consistently reduced flight speed and distance run during escape in the MDTB, and increased contacts of the mouse subjects with an anesthetized rat (Pobbe & Zangrossi 2005). However, the 5-HT1a antagonist WAY-100635, which impaired escape in the ETM (Pobbe & Zangrossi 2005), did not affect defensive behaviors in the MDTB (Pobbe et al. 2011).
In summary, these findings suggest that flight is modulated by some relatively specific neurotransmitter mechanisms involving particular brain circuitry. They indicate relatively good agreement among the several measures of flight that have been devised to differentiate flight from other defensive behaviors or specifically to measure responsiveness of flight to drugs and other variables that affect the modulation of panic. These findings also support the concept of a specific link between flight and panic.
13.3 Conclusions
Nearly a century of research using increasingly sophisticated and multifaceted neuroscience methodologies has produced a great deal of information on endocrine, neuroanatomic, and neurochemical systems involved in defensive behaviors, including flight/escape. The components of these systems that specifically control flight/panic, as distinct from other defensive behaviors, have received a great deal of recent attention and analysis due to an increasingly well-established association of flight/escape with panic. However, research on escape decisions based on costs and benefits of fleeing such as flight initiation distance, distance fled, and hiding time in refuge, are also needed to determine the physiological underpinnings of escape decisions that are the foci of economic models of escape (see Chapter 2).
References
Apfelbach, R., Blanchard, C. D., Blanchard, R. J., Hayes, R. A. & Mcgregor, I. S. (2005). The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neuroscience Biobehavioral Reviews, 29, 1123-1144.
Bard, P. (1928). A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal Physiology, 84, 490-410.
Beijamini, V. & Andreatini, R. (2003). Effects of Hypericum perforatum and paroxetine in the mouse defense test battery. Pharmacology Biochemistry and Behavior, 74, 1015-1024.
Bittencourt, A. S., Carobrez, A. P., Zamprogno, L. P., Tufik, S. & Schenberg, L. C. (2004). Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: Role of N-methyl-D-aspartic acid glutamate receptors. Neuroscience, 125, 71-89.
Blanchard, D. C. (1997). Stimulus, environmental and pharmacological control of defensive behaviors. In Bouton, M. & Fanselow, M. S. (eds.) Learning, Motivation and Cognition. The Functional Behaviorism of Robert C. Bolles. Washington DC: American Psychological Association.
Blanchard, R. J. & Blanchard, D. C. (1989). Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology, 103, 70-82.
Blanchard, D. C. & Blanchard, R. J. (2008). Defensive behaviors, fear and anxiety. In Blanchard, R. J., Blanchard, D. C., Griebel, G. & Nutt, D. J. (eds.) Handbook of Anxiety and Fear. Amsterdam: Elsevier Academic Press.
Blanchard, R. J., Taukulis, H. K., Rodgers, R. J., Magee, L. K. & Blanchard, D. C. (1993). Yohimbine potentiates active defensive responses to threatening stimuli in Swiss-Webster mice. Pharmacology Biochemistry and Behavior, 44, 673-681.
Blanchard, R. J., Kaawaloa, J. N., Hebert, M. A. & Blanchard, D. C. (1999). Cocaine produces panic-like flight responses in mice in the mouse defense test battery. Pharmacology Biochemistry and Behavior, 64, 523-528.
Blanchard, D. C., Griebel, G. & Blanchard, R. J. (2001). Mouse defensive behaviors: Pharmacological and behavioral assays for anxiety and panic. Neuroscience and Biobehavioral Reviews, 25, 205-218.
Blanchard, D. C., Griebel, G. & Blanchard, R. J. (2003a). The Mouse Defense Test Battery: Pharmacological and behavioral assays for anxiety and panic. European Journal of Pharmacology, 463, 97-116.
Blanchard, D. C., Markham, C., Yang, M. et al. (2003b). Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behavioral Neuroscience, 117, 360-368.
Blanchard, D. C., Litvin, Y., Pentkowski, N. S. & Blanchard, R. J. (2009). Defense and aggression. In Berntson, G. G. & Cacioppo, J. T. (eds.) Handbook of Neuroscience for the Behavioral Sciences. Hoboken, NJ: John Wiley & Sons.
Blanchard, D. C., Griebel, G., Pobbe, R. & Blanchard, R. J. (2011). Risk assessment as an evolved threat detection and analysis process. Neuroscience Biobehavioral Reviews, 35, 991-998.
Borelli, K. G., Ferreira-Netto, C., Coimbra, N. C. & Brandao, M. L. (2005). Fos-like immunoreactivity in the brain associated with freezing or escape induced by inhibition of either glutamic acid decarboxylase or GABAA receptors in the dorsal periaqueductal gray. Brain Research, 1051, 100-111.
Bourin, M., Baker, G. B. & Bradwejn, J. (1998). Neurobiology of panic disorder. Journal of Psychosomatic Research, 44, 163-180.
Bovier, P., Broekkamp, C. L. & Lloyd, K. G. (1982). Enhancing GABAergic transmission reverses the aversive state in rats induced by electrical stimulation of the periaqueductal grey region. Brain Research, 248, 313-320.
Brandao, M. L., De Aguiar, J. C. & Graeff, F. G. (1982). GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacology Biochemistry and Behavior, 16, 397-402.
Cannon, W. B. (1915). Bodily Changes in Pain, Hunger, Fear and Rage. New York, NY: D. Appleton & Company.
Cannon, W. B. (1927). The James-Lange theory of emotion: A critical examination and an alternative theory. American Journal of Psychology, 39, 106-124.
Canteras, N. S. (2002). The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacology Biochemistry and Behavior, 71, 481-491.
Canteras, N. S. & Graeff, F. G. (2014). Executive and modulatory neural circuits of defensive reactions: Implications for panic disorder. Neuroscience Biobehavioral Reviews, 46, 352-364.
Canteras, N. S., Kroon, J. A., Do-Monte, F. H., Pavesi, E. & Carobrez, A. P. (2008). Sensing danger through the olfactory system: The role of the hypothalamic dorsal premammillary nucleus. Neuroscience Biobehavioral Review, 32, 1228-1235.
Cardoso, S. H., Coimbra, N. C. & Brandao, M. L. (1994). Defensive reactions evoked by activation of NMDA receptors in distinct sites of the inferior colliculus. Behavioral Brain Research, 63, 17-24.
Carrasco, G. A. & Van De Kar, L. D. (2003). Neuroendocrine pharmacology of stress. European Journal of Pharmacology, 463, 235-272.
Cezario, A. F., Ribeiro-Barbosa, E. R., Baldo, M. V. & Canteras, N. S. (2008). Hypothalamic sites responding to predator threats: The role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. European Journal of Neuroscience, 28, 1003-1015.
Coimbra, N. C. & Brandao, M. L. (1993). GABAergic nigro-collicular pathways modulate the defensive behaviour elicited by midbrain tectum stimulation. Behavioral Brain Research, 59, 131-139.
Cox, B. J., Norton, G. R., Swinson, R. P. & Endler, N. S. (1990). Substance abuse and panic-related anxiety: A critical review. Behavioral Research and Therapy, 28, 385-393.
De Bortoli, V. C., Nogueira, R. L. & Zangrossi, H., Jr. (2006). Effects of fluoxetine and buspirone on the panicolytic-like response induced by the activation of 5-HT1A and 5-HT2A receptors in the rat dorsal periaqueductal gray. Psychopharmacology, 183, 422-428.
De Paula Soares, V. & Zangrossi, H., Jr. (2004). Involvement of 5-HT1A and 5-HT2 receptors of the dorsal periaqueductal gray in the regulation of the defensive behaviors generated by the elevated T-maze. Brain Research Bulletin, 64, 181-188.
Deakin, J. F. & Graeff, F. G. (1991). 5-HT and mechanisms of defence. Journal of Psychopharmacology, 5, 305-315.
Diamond, D. M., Bennett, M. C., Fleshner, M. & Rose, G. M. (1992). Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus, 2, 421-430.
Dielenberg, R. A., Hunt, G. E. & Mcgregor, I. S. (2001). “When a rat smells a cat”: The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience, 104, 1085-1097.
Du Vigneaud, V., Ressler, C. & Trippett, S. (1953). The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. Journal of Biological Chemistry, 205, 949-957.
Ferreira-Netto, C., Borelli, K. G. & Brandao, M. L. (2005). Neural segregation of Fos-protein distribution in the brain following freezing and escape behaviors induced by injections of either glutamate or NMDA into the dorsal periaqueductal gray of rats. Brain Research, 1031, 151-163.
Fokkema, D. S. & Koolhaas, J. M. (1985). Acute and conditioned blood pressure changes in relation to social and psychosocial stimuli in rats. Physiology and Behavior, 34, 33-38.
Graeff, F. G. (1990). Brain defence systems and anxiety. In Roth, M., Burrow, G. D. & Noyes, R. (eds.) Handbook of Anxiety, Vol. 3, 307-357. Amsterdam: Elsevier.
Graeff, F. G. (1997). Serotonergic systems. Psychiatric Clinics of North America, 20, 723-739.
Graeff, F. G., Viana, M. B. & Tomaz, C. (1993). The elevated T maze: A new experimental model of anxiety and memory: effect of diazepam. Brazilian Journal of Medical and Biological Research, 26, 67-70.
Griebel, G., Blanchard, D. C., Agnes, R. S. & Blanchard, R. J. (1995a). Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology, 120, 57-66.
Griebel, G., Blanchard, D. C., Jung, A. et al. (1995b). Further evidence that the mouse defense test battery is useful for screening anxiolytic and panicolytic drugs: Effects of acute and chronic treatment with alprazolam. Neuropharmacology, 34, 1625-1633.
Griebel, G., Blanchard, D. C. & Blanchard, R. J. (1996). Predator-elicited flight responses in Swiss-Webster mice: An experimental model of panic attacks. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 20, 185-205.
Gross, C. T. & Canteras, N. S. (2012). The many paths to fear. Nature Reviews Neuroscience, 13, 651-658.
Hahn, J. D. & Swanson, L. W. (2012). Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. Journal of Comparative Neurology, 520, 1831-1890.
Herman, J. P., Ostrander, M. M., Mueller, N. K. & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29, 1201-1213.
Hogg, S., Michan, L. & Jessa, M. (2006). Prediction of anti-panic properties of escitalopram in the dorsal periaqueductal grey model of panic anxiety. Neuropharmacology, 51, 141-145.
Huhman, K. L., Bunnell, B. N., Mougey, E. H. & Meyerhoff, J. L. (1990). Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiology and Behavior, 47, 949-956.
Joels, M. & De Kloet, E. R. (1992). Control of neuronal excitability by corticosteroid hormones. Trends in Neurosciences, 15, 25-30.
Johnson, M. R., Lydiard, R. B. & Ballenger, J. C. (1995). Panic disorder. Pathophysiology and drug treatment. Drugs, 49, 328-344.
Johnson, P. L., Molosh, A., Fitz, S. D., Truitt, W. A. & Shekhar, A. (2012). Orexin, stress, and anxiety/panic states. Progress in Brain Research, 198, 133-161.
Karatsoreos, I. N. & McEwen, B. S. (2013). Resilience and vulnerability: A neurobiological perspective. F1000 Prime Reports, 5, 13.
Klein, D. F. (1993). False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry, 50, 306-317.
Litvin, Y. & Pfaff, D. W. (2013). The involvement of oxytocin and vasopressin in fear and anxiety. In Choleris, E., Pfaff, D. W. & Kavaliers, M. (eds.) Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge: Cambridge University Press.
Litvin, Y., Pentkowski, N. S., Pobbe, R. L., Blanchard, D. C. & Blanchard, R. J. (2008). Unconditioned models of fear and anxiety. In Blanchard, R. J., Blanchard, D. C., Griebel, G. & Nutt, D. J. (eds.) Handbook of Anxiety and Fear. Amsterdam: Elsevier Academic Press.
Lowry, C. A., Johnson, P. L., Hay-Schmidt, A., Mikkelsen, J. & Shekhar, A. (2005). Modulation of anxiety circuits by serotonergic systems. Stress, 8, 233-246.
Lupien, S. J. & McEwen, B. S. (1997). The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews, 24, 1-27.
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873-904.
McGaugh, J. L. & Roozendaal, B. (2002). Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology, 12, 205-210.
Melo, L. L., Cardoso, S. H. & Brandao, M. L. (1992). Antiaversive action of benzodiazepines on escape behavior induced by electrical stimulation of the inferior colliculus. Physiology and Behavior, 51, 557-562.
Micheau, J., Destrade, C. & Soumireu-Mourat, B. (1984). Time-dependent effects of posttraining intrahippocampal injections of corticosterone on retention of appetitive learning tasks in mice. European Journal of Pharmacology, 106, 39-46.
Mochcovitch, M. D. & Nardi, A. E. (2010). Selective serotonin-reuptake inhibitors in the treatment of panic disorder: A systematic review of placebo-controlled studies. Expert Review of Neurotherapeutics, 10, 1285-1293.
Motta, S. C., Goto, M., Gouveia, F. V. et al. (2009). Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proceedings of the National Academy of Sciences, 106, 4870-4875.
O’Keefe, J. & Nadel, L. (1978). The Hippocampus as a Cognitive Map. Oxford: Oxford University Press.
Pentkowski, N. S., Blanchard, D. C., Lever, C., Litvin, Y. & Blanchard, R. J. (2006). Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. European Journal of Neuroscience, 23, 2185-2196.
Petrovich, G. D., Canteras, N. S. & Swanson, L. W. (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews, 38, 247-289.
Pobbe, R. L. & Zangrossi, H., Jr. (2005). 5-HT(1A) and 5-HT(2A) receptors in the rat dorsal periaqueductal gray mediate the antipanic-like effect induced by the stimulation of serotonergic neurons in the dorsal raphe nucleus. Psychopharmacology, 183, 314-321.
Pobbe, R. L., Zangrossi, H., Jr., Blanchard, D. C. & Blanchard, R. J. (2011). Involvement of dorsal raphe nucleus and dorsal periaqueductal gray 5-HT receptors in the modulation of mouse defensive behaviors. European Neuropsychopharmacology, 21, 306-315.
Quintino-Dos-Santos, J. W., Muller, C. J., Bernabe, C. S. et al. (2014). Evidence that the periaqueductal gray matter mediates the facilitation of panic-like reactions in neonatally-isolated adult rats. PLoS One, 9, e90726.
Risold, P. Y. & Swanson, L. W. (1995). Evidence for a hypothalamothalamocortical circuit mediating pheromonal influences on eye and head movements. Proceedings of the National Academy of Sciences U S A, 92, 3898-3902.
Romero, L. M. (2004). Physiological stress in ecology: Lessons from biomedical research. Trends in Ecology and Evolution, 19, 249-255.
Roncon, C. M., Biesdorf, C., Coimbra, N. C. et al. (2013). Cooperative regulation of anxiety and panic-related defensive behaviors in the rat periaqueductal grey matter by 5-HT1A and mu-receptors. Journal of Psychopharmacology, 27, 1141-1148.
Roozendaal, B., Van Der Zee, E. A., Hensbroek, R. A. et al. (1997). Muscarinic acetylcholine receptor immunoreactivity in the amygdala-II. Fear-induced plasticity. Neuroscience, 76, 75-83.
Roozendaal, B., Hahn, E. L., Nathan, S. V., De Quervain, D. J. & Mcgaugh, J. L. (2004). Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. Journal of Neuroscience, 24, 8161-8169.
Rosen, J. B. (2004). The neurobiology of conditioned and unconditioned fear: A neurobehavioral system analysis of the amygdala. Behavioral and Cognitive Neuroscience Reviews, 3, 23-41.
Spiess, J., Rivier, J., Rivier, C. & Vale, W. (1981). Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proceedings of the National Academy of Sciences, 78, 6517-6521.
Staples, L. G., McGregor, I. S., Apfelbach, R. & Hunt, G. E. (2008). Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience, 151, 937-947.
Thaker, M., Vanak, A. T., Lima, S. L. & Hews, D. K. (2010). Stress and aversive learning in a wild vertebrate: The role of corticosterone in mediating escape from a novel stressor. American Naturalist, 175, 50-60.
Tuppy, H. (1953). The amino-acid sequence in oxytocin. Biochimica et Biophysica Acta, 11, 449-450.
Turner, R. A., Pierce, J. G. & Du, V. V. (1951). The purification and the amino acid content of vasopressin preparations. Journal of Biological Chemistry, 191, 21-28.
Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213, 1394-1397.
Viana, M. B., Tomaz, C. & Graeff, F. G. (1994). The elevated T-maze: a new animal model of anxiety and memory. Pharmacology Biochemistry & Behavior, 49, 549-554.
Viken, R. J., Knutson, J. F. & Johnson, A. K. (1989). Effects of behavior and social condition on cardiovascular response to footshock stress. Physiology and Behavior, 46, 961-966.
Yang, M., Farrokhi, C., Vasconcellos, A., Blanchard, R. J. & Blanchard, D. C. (2006). Central infusion of Ovine CRF (oCRF) potentiates defensive behaviors in CD-1 mice in the Mouse Defense Test Battery (MDTB). Behavioral Brain Research, 171, 1-8.
Zangrossi, H., Jr. & Graeff, F. G.(2014). Serotonin in anxiety and panic: Contributions of the elevated T-maze. Neuroscience Biobehavioral Reviews, 46, 397-406.
Zanoveli, J. M., Nogueira, R. L. & Zangrossi, H., Jr. (2003). Serotonin in the dorsal periaqueductal gray modulates inhibitory avoidance and one-way escape behaviors in the elevated T-maze. European Journal of Pharmacology, 473, 153-161.