Fasting for Life: Medical Proof Fasting Reduces Risk of Heart Disease, Cancer, and Diabetes - Francis E. Umesiri (2016)
Part 1. The Science of Fasting
Chapter 8. BOOST YOUR BRAIN
I fast for greater physical and mental efficiency.
—PLATO1
DOES FASTING IMPROVE BRAIN HEALTH AND function? It turns out that a significant number of research studies indicate that fasting can do just this. There is some evidence that calorie restriction or intermittent fasting offers protection against age-related neuronal loss and neurodegenerative disorders such as dementia, Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease. Caloric restriction and intermittent fasting have also been shown to reduce the risk of stroke and depression. These are significant statements to make about the role of fasting in improving brain health. In a 2014 TED talk Mark Mattson—chief of the Laboratory of Neurosciences at the National Institute on Aging—commented, “We think the reason—the main take-home message of this talk is that fasting is a challenge to your brain and your brain responds to that challenge of not having food by activating adaptive stress response pathways that help your brain cope with stress and risk.”2
In this chapter I will highlight some of the studies making these correlations between fasting and cognitive function and mood. But first, a brief crash course on the molecular basis for the role of fasting in improving brain health. In other words, in what ways does fasting contribute to health function at the biomolecular level? Although some fairly technical terms are involved, you don’t need a degree in biochemistry or pharmacology to understand this. In chapter 4 I reviewed some of the molecular mechanisms involved in health benefits from fasting in general; namely, hormesis (activation of adaptive cellular stress responses), improved mitochondrial function, and antioxidant activity.
While all those mechanisms also apply to neuroprotection, in this chapter I will focus more on other specific cellular and molecular mechanisms of fasting in improving brain health. These mechanisms include increased neurotrophic factor activity and improved neurogenesis, sirtuin activity, neuronal autophagy, protein chaperone activity, ketogenic bodies, and anti-inflammatory effects. But for the purposes of helping readers better understand this section, I will focus on three of those mechanisms: neurotrophic factor activity and neurogenesis, neuronal autophagy, and ketone bodies.3
Neurotrophic factors
Neurotrophic factors, sometimes referred to simply as neurotrophins, are growth factors that promote the survival and growth of neurons. This class of proteins promotes growth and development of neurons in the central nervous system and peripheral nervous system. There are about four subclasses of these structurally related factors:
✵ Nerve growth factor (NGF)
✵ Brain-derived neurotrophic factor (BDNF)
✵ Neurotrophin-3 (NT-3)
✵ Neurotrophin-4 (NT-4)4
Their mode of action is to prevent initiation of neuronal-programmed cell death, thereby allowing neurons to survive. Neurotrophic factors also induce and facilitate differentiation of precursor cells to form new neurons. While all these growth factors play important roles, the focus here is the role of brain-derived neurotrophic factor in brain function. Studies indicate now that degenerative diseases of the nervous system may result from insufficient supply of neurotrophic factors, especially BDNF. It is true that a majority of neurons in the human brain are formed prenatally; the hippocampus is one of the few parts of the adult brain that still retain the ability to grow new neurons. This occurs through a process known as neurogenesis. And it is this process that is facilitated by BDNF and other growth factors. BDNF is present in the hippocampus, cortex, cerebellum, and basal forebrain.
A key fact about the hippocampus: it is the region of the brain known to regulate learning, memory, and mood. In addition, the hippocampus is very sensitive and responsive to external stimuli, meaning that the hippocampus can easily activate adaptive response to cellular stress.5 In part, this ability of the brain to change in response to different stimuli is known as brain plasticity.
Many studies have shown some evidence of decreased expression of BDNF in neurological diseases, some of which I will examine in more detail. While studies in this area are ongoing, suffice it to say that numerous reports demonstrate that reduced BDNF levels correlates with several neurodegenerative disorders. Several studies have shown that fasting leads to increased levels of BDNF, and therefore offers some neuroprotection against neurodegenerative diseases implicated in low levels of BDNF, such as Alzheimer’s and Huntington’s disease.
Neuronal autophagy
To get a better grasp of “brain power,” consider its functions and vast network of neurons. Let’s review some essentials about the central nervous system (CNS), the brain and the spinal cord:
✵ The CNS has about 1,000,000,000,000 neurons and 1,000,000,000,000,000 synapses; 62,000 miles of myelinated axons; and 100,000 miles of dendrites; it also has up to 15,000 connections per cell.
✵ The average neuron may have about 1,000 synapses.
✵ The average axon may synapse on about 1,000 neurons.
✵ Each of the 100 billion neurons may have the processing capacity of a medium-sized computer, computing about a thousand multiplications and additions every ten milliseconds.6
There are literally billions of neuronal activities going on at any given time. Dendrites serve as a source of informational input to the neuron while synapses serve to relay information between neurons (Figure 1). The axon is responsible for transferring proteins and organelles over significant distances in the nervous system, making the quality control of proteins critical for proper neuronal function. In addition, synapses require high-energy demand and protein turnover for proper functioning. More importantly, neurons are postmitotic and do not replicate, meaning they are predisposed to accumulate toxic proteins and impaired organelles.7
While neurogenesis, facilitated by neurotropic factors, involves preventing death of needed neurons, neuronal autophagy means facilitating death of neurons that are no longer needed or that have become loaded with toxic proteins. Due to the importance of tightly controlling proteins and organelles in the neurons, neuronal autophagy seems to be regulated separately from that of non-neuronal cells.8
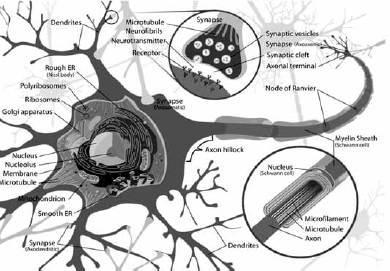
Figure 1: Image of a Neuron9
What does this ability of the body to self-destroy damaged neurons that have accumulated toxic proteins or dysfunctional organelles have to do with neurodegenerative diseases—namely, such serious diseases as Alzheimer’s disease, Parkinson’s, Huntington’s, and amyotrophic lateral sclerosis (often called Lou Gehrig’s disease)? As it turns out, quite a bit. Intracellular aggregation of proteins and damaged organelles are common features of neurodegenerative diseases.10 This means that in the course of these diseases, toxic protein aggregates that have accumulated within specific types of neurons lead to their malfunctioning and ultimately neuronal death.
In essence, any process that increases the ability of the body to effectively and selectively destroy those damaged neurons (known as autophagy) improves the prognosis for neurodegenerative diseases or reduces the risk of their early onset. Interestingly one of those experimentally proven interventions that seem to increase neuronal autophagy is fasting. In a study conducted in 2010, researchers showed that fasting in mice “causes a rapid and profound upregulation of autophagy in the brain.”11
The experiment was conducted on six-to-seven-week-old male GFP-LC3 mice, which ate a food-restricted diet for twenty-four or forty-eight hours. This process is known to induce fatty change and autophagy in the liver. Scientists freely provided water to these experimental mice. Then they euthanized these mice and non-fasting control mice. Sections of their livers and brains were prepared, stained, photographed, and analyzed for autophagosomes. Calorie restriction is a well-known scientific way to induce autophagy and upregulates autophagy in many organs including the liver. For years many scientists believed that perhaps the brain is able to escape this effect—probably because it is a metabolically privileged site. But this study shows that autophagy not only works in the brain, but also that food restriction stimulates and upregulates it within the brain as well.12
Ketone bodies
Ketone bodies are a group of three compounds that are produced as by-products from fatty acid catabolism in the liver and kidney. They are used as a source of energy, especially for the brain. Under normal circumstances, the brain derives most of its energy from glucose metabolism. However, during fasting periods, glucose level is depleted, so the brain relies more on availability of ketone bodies for energy supply. The three compounds are β-hydroxybutyrate and acetoacetate, and acetone.13 Although they are called “bodies,” they are actually chemical compounds that are soluble in water. As ketone bodies build up in the blood during or immediately after fasting, they cross the blood-brain barrier through monocarboxylic acid transporters, which are proton-linked transporters. Transported ketone bodies then enter neurons either by diffusion or through monocarboxylic acid transporters.14Expectedly fasting increases the permeability of the blood-brain barrier to ketones and improves the expression of monocarboxylic acid transporters.
So, what do ketone bodies from fats have to do with brain health? Quite a bit. The brain functions through the ability of excited neurons to transmit signals and process information. So, while neuron excitation is a good thing, overexcitation is bad for neurons, since it tends to destroy them. This means that the brain has to constantly regulate excitation and balance it with a level of inhibition. Two neurotransmitters play this regulatory role. On one hand is glutamate, which is the excitatory neurotransmitter. On the other is gamma-aminobutyric acid (GABA), which inhibits excitation. Significantly in seizures, stroke, and neurodegenerative diseases there is more glutamate excitation going on, which is a problem. It can lead to calcium-dependent neuronal injury and death, through generation of reactive oxygen species and damage to mitochondrial bioenergetic function.15
So, here is where ketone bodies come in. As far back as the 1930s, studies showed that directly injecting ketone bodies into rabbits stopped chemically induced seizures through inhibition of glutamate release. For decades the mechanism was unknown. However, recent studies involving hippocampus neurons have shown that ketone bodies directly inhibited glutamate-mediated excitation. On the other hand, ketone bodies have also been shown to increase GABA in synapses of rats and even in certain human brains.16
Also, another study showed that a combination of β-hydroxybutyrate and acetoacetate at very low millimolar range decreased the production of reactive oxygen species by complex I of the mitochondrial respiratory chain.17 It is important to note that ketone bodies prevented neuronal injury and apoptosis caused by hydrogen peroxide or by the glutathione oxidant.18 All of this means that ketone bodies, available either through fasting or by ingesting a ketogenic diet, have neuroprotective effects.
Empirical evidence
Now that I have established a framework for understanding some of the molecular mechanism of action of fasting in improving brain health, I want to examine studies related to depression, mild memory loss, Alzheimer’s disease, and stroke.
Fasting and depression
The federal Centers for Disease Control and Prevention (CDC) estimates that about one in ten Americans suffer from depression at any given time.19 According to the CDC, depression ranges from mild or moderate depressive disorder (“other depression”) to major depression. Referencing a study, its report says: “Participants were considered to have major depression if, for ‘more than half the days,’ they met at least five of the eight criteria, including at least one of the following: 1) ‘little interest or pleasure in doing things’ or 2) ‘feeling down, depressed, or hopeless.’ The [other] criteria were: 3) ‘trouble falling asleep or staying asleep or sleeping too much,’ 4) ‘feeling tired or having little energy,’ 5) ‘poor appetite or overeating,’ 6) ‘feeling bad about yourself or that you were a failure or let yourself or your family down,’ 7) ‘trouble concentrating on things, such as reading the newspaper or watching television,’ and 8) ‘moving or speaking so slowly that other people could have noticed … or the opposite: being so fidgety or restless that you were moving around a lot more than usual.’ Participants were considered to have ‘other depression’ if they met two, three, or four of the eight criteria [for major depression listed above], including at least one of the following 1) ‘little interest or pleasure in doing things’ or 2) ‘feeling down, depressed, or hopeless.’”20
According to 2009-2010 estimates, about eight million Americans a year are rushed to hospitals or emergency rooms in ambulances with depression as the major diagnosis, with more than 39,000 suicides resulting from depressive disorders. Antidepression medications represent the third most common prescription taken by Americans of all ages; for the most recently available data for the period 2005-2008, the rate of antidepressant use in the United States increased about 400 percent.21 Without a doubt, depression is a major health issue. Additionally the fact that depression is associated with certain chronic diseases such as obesity and stroke makes it an important health risk that the nation must tackle.
So, what does fasting have to do with depression? For many years clinical researchers have found a positive correlation between fasting and mood improvement in patients. The staff at the department of internal and complementary medicine at Immanuel Hospital in Berlin, Germany, has done quite a bit of work in this area. In 2006 they conducted a study (among others) that showed patients put on a fasting regimen experienced improvement in mood.22 Conducted in a nutritional ward, doctors placed thirty-six patients on an eight-day modified fast (300 kcal/day). They then took daily measurements of ratings of mood, weight, and levels of leptin and cortisol four times within a two-week study period. Fasters showed a more pronounced decrease of leptin (58 percent vs. 20 percent; P < 0.001) and a 17 percent increase in levels of cortisol, the “stress hormone” (P < 0.001). Mood ratings increased significantly toward the later phase of fasting (P < 0.01) but were not related to weight loss, leptin depletion or cortisol increase. The study concluded that fasting induces specific mood enhancement, but indicates that the physiological pathway may not be due to cortisone increase or leptin reduction.23 In fact, in an earlier study in 2002 involving the effects of fasting (250 kcal/day) for two weeks in fifty-two patients with chronic pain and metabolic syndrome, researchers found that over 80 percent of fasters showed a rapid decrease in depression and anxiety scores, with an average weight loss of 14.6 pounds.24
In another human study conducted in Malaysia, thirty-two men with an average age of 58.8 ±5.1 years were placed on a 25 percent calorie restriction in addition to two days per week of religiously based fasting. At the end of the three-month study those men scored lower in tests for depression.25
So, how does fasting work to improve mood and reduce depression? Remember BDNF, that brain-derived neurotrophic factor? BDNF has been demonstrated to be an important biomarker for major depression. Several clinical studies have shown that serum levels of BDNF are significantly lower in patients with major depressive disorder. What’s more, antidepressant treatments reverse this effect. It has been shown that antidepressants appear to work by increasing the level of BDNF in the hippocampus.26 In fact, a study by two researchers showed that direct infusion of BDNF into the hippocampus is enough to elicit an antidepressant-like effect in animal models of depression.27And to further show that BDNF has a direct role in antidepressant efficacy, mouse models in which the BDNF gene had been mutated or deleted completely did not even respond to antidepressants.
BDNF also produces antidepression effects in the brain and promotes neurogenesis.28 Today the scientific community seems to have reached a consensus that neurotrophic factors—especially BDNF—play an important role in signaling pathways in the hippocampus and prefrontal cortex involved in ameliorating depression. Equally important is the finding from these studies that stress suppresses BDNF levels in the hippocampus and prefrontal cortex. To simplify: these studies show that almost all antidepressant treatments increase BDNF synthesis and improve BDNF signaling within the brain’s hippocampus and prefrontal cortex. This means that increasing BDNF within the brain correlates with improved mood and, hence, decreased anxiety and depressive disorder.
You may wonder if any evidence indicates that fasting improves levels of BDNF in depression patients; the answer is yes. Studies suggest that the beneficial role of fasting on depression is due to increased BDNF levels observed during fasting. Fasting has been shown to cause an increase in BDNF involved in the regulation of serotonin metabolism, synaptic plasticity, improved cognitive function, and increasing the brain’s ability to resist aging.29 Another mechanism thought to aid mood during fasting is the increased production of ketone bodies, which have been demonstrated to improve mood and decrease pain sensation.30
It is also possible that some of the effects of fasting on mood may be explained simply by hormesis. For example, scientists have surmised that prolonged fasting acts as a strong physiological stimulus equivalent to a biological stress, activating the hypothalamic-pituitary-adrenal axis (HPA, the “stress axis”), which produces several adaptive hormones and neurotransmitters.31 Although the precise biological mechanism of activation may be unclear, it is believed to include reduced availability of cerebral glucose, and reduced insulin and leptin levels.
Taken as a whole, these studies suggest that fasting induces a range of biochemical and physiological processes that elicit a beneficial effect in mood improvement, leading to observed improvement in depressive symptoms. To be clear: these studies are in their early stages. There are still no credible, randomized, controlled clinical trials to study the effectiveness of fasting on major depressive disorder. Most of the current studies are on animal models. So, until we have more than a few exploratory studies involving human subjects, these studies need to be treated with a dose of skepticism.
Still, every biomedical breakthrough in humans has followed the same sound scientific experimentation and peer-review process starting with various animal models. While I am not suggesting you embark on a protracted fast to cure major depressive disorder without consulting your doctor, initial results are that sustained, preventive fasting does offer benefits, even mood improvement.
Fasting and memory improvement
As far back as 1987 researchers demonstrated that calorie restriction in mice resulted in improved memory and motor coordination. One set of mice ate a normal diet (95 kcal/week), while a second set of mice followed a limited diet (55 kcal/week) for about thirty-five months. The scientists observed that the mice on a restricted diet saw less age-related decline in motor coordination and learning, with their motor coordination enhanced in comparison to the group on a normal diet.32
In chapter 3 I mentioned the 2009 study by internal medicine researchers at the University of Münster in Germany, who set out to investigate if such beneficial effects can be observed in humans. To give you a little more detail, they enrolled fifty adults ranging in age from fifty to eighty. In this interventional study they divided the participants into three groups:
✵ One group (the control) followed a normal diet as before.
✵ A second group had a 30 percent reduction in calorie intake.
✵ The third group ate normally, but with a 20 percent increase in unsaturated fatty acids (with no overall increase in fat intake).
The doctors wanted to see if calorie reduction and/ or increase in unsaturated fatty acids could lead to improvement in memory. The fasting group showed a 20 percent increase in memory scores/ability (P < 0.001) than the group that didn’t reduce calorie intake. They also noticed that this increase in memory ability correlated to increased insulin sensitivity and reduced inflammatory activity.33
Increased oxidative stress and impaired energy metabolism, among other factors already discussed, are believed to contribute significantly to neuronal dysfunction and death resulting in memory loss and other neurodegenerative diseases. This interventional study provided some evidence that caloric restriction does have beneficial effects on memory performance in healthy elderly people. This occurs, in part, by improving glucose metabolism, resulting in higher insulin sensitivity and inducement of a range of other cellular adaptive processes.34
Alzheimer’s disease
Alzheimer’s disease has been identified as the most common form of dementia. Dementia is a general term used to describe the loss of memory and other intellectual abilities serious enough to interfere with daily life. Alzheimer’s disease is believed to account for between 60 and 80 percent of all dementia cases. According to the Alzheimer’s Association about 5.3 million Americans had Alzheimer’s disease in 2015; approximately 200,000 individuals were younger than age sixty-five. Women, pay attention: females are particularly at risk. Almost two-thirds of Americans living with Alzheimer’s are women. The number is estimated at 3.2 million, compared to 1.8 million men. In fact, the average woman has a greater chance of developing Alzheimer’s (one in six) than breast cancer (one in eleven).35
It is not clear why women seem to be at greater risk, but that is reality. Alzheimer’s is the sixth leading cause of death in the nation, with researchers estimating this disease claims up to 500,000 lives annually. In other words, many of those people wouldn’t have died if they did not have Alzheimer’s. Between 2000 and 2010 mortality rate for other diseases decreased, but mortality from Alzheimer’s increased by 68 percent within the same span of time. In addition, Alzheimer’s is the most expensive medical condition to treat. Unless a major medical breakthrough occurs as baby boomers age, the number of Americans aged sixty-five and above with Alzheimer’s is projected to skyrocket to about sixteen million by 2050.36
Unlike what most people are made to believe, Alzheimer’s is not necessarily a disease caused by old age, as though it were a “normal” part of aging. Still, the best known risk is aging. The brain of someone with Alzheimer’s is often associated with certain biochemical and physiological changes; mainly, a buildup of extracellular β-amyloid protein, which results in plaque buildup. In addition, there is an aggregation of hyperphosphorylated forms of special structural protein, called tau protein. This leads to biological tangles, called neurofibrillary tangles, and shrinking of the brain.37
The Alzheimer’s Association’s website has a user-friendly, interactive tool about the brain that you may find useful. Think plaques and a twisted mass of tangles in the brain, which can’t be good for such sensitive tissue. Synapses, those points where “messages” are relayed from one neuron to another in order to communicate with the body, get affected the most by this buildup of plaques and tangles. They become easily impaired and are ultimately damaged, leading to a range of neurodegenerative conditions.38 While there is no known cure for this disease, it can be managed medically. That leaves scientists to examine behavioral factors, such as diet and health practices, that could help delay or reduce the risk of Alzheimer’s onset. It is in this light that several studies have been conducted to investigate the role—if any—that fasting (calorie restriction or intermittent fasting) may have on reducing the risk of Alzheimer’s or even delaying its onset significantly.
Is there any evidence that fasting can reduce the risk of Alzheimer’s disease, either from animal or human studies? Fortunately yes. In 2007 researchers using a transgenic mouse model of Alzheimer’s disease demonstrated that a 30 percent calorie restriction resulted in production of more genes associated with neurogenesis, prevented the hippocampus from atrophy, and reduced the production of genes known to stimulate inflammation.39 In another animal study in 2007 involving mice genetically engineered to have Alzheimer’s disease, researchers showed that a 40 percent calorie restriction or intermittent fasting for fourteen months significantly reduced or delayed cognitive decline.40 Interestingly the improvement in cognitive function is also correlated with significantly reduced levels of β-amyloid protein and phospho-tau (remember those two implicated in causing brain plaque and tangles?).
While there is evidence of improvement in animal models, how about human studies? Clinical studies involving humans are scarce and only beginning to emerge. Still, there are epidemiological studies, which refers to the presence of diseases in large population. One such study in Sweden, completed in 2005, investigated the relation between midlife body mass index and the risk of vascular risk factors, and subsequent dementia and Alzheimer’s. Researchers studied more than 1,400 people and followed up on them for twenty-one years.41 At the end of the study they concluded that being obese at midlife (a BMI greater than 30kg/m2) was directly associated with higher risk of dementia and Alzheimer’s disease in particular, even after adjusting for sociodemographic variables, midlife blood pressure, smoking, cholesterol level, and other factors.42
In another study conducted in New York, researchers learned that low dietary energy intake was directly associated with decreased incidence of Alzheimer’s disease and Parkinson’s disease.43 As you can imagine, these human studies are not yet sufficient to draw any far-reaching conclusions as it specifically relates to ameliorating Alzheimer’s in humans. However, studies from animal models and other brain health studies show clearly that fasting does have a beneficial effect on memory and results in reduced levels of plaques and tangles associated with Alzheimer’s disease.
Fasting and stroke
After Alzheimer’s, the next most common form of dementia is vascular dementia, which occurs after a stroke. So, it is worth discussing stroke in general, and the possible role of fasting in reducing these risks. Stroke is now the fifth leading cause of death in the nation and a major cause of disability in adults. Stroke is a disease that affects the arteries leading to the brain, or arteries within the brain. According to the National Institute of Neurological Disorders and Stroke (NINDS), an arm of the National Institutes of Health, stroke occurs when there is a sudden interruption in blood supply to the brain. Or when a blood vessel within the brain busts and spills its blood onto brain cells. Since brain cells cannot survive without oxygen and nutrients carried by the blood, they die.
There are two kinds of stroke: ischemic stroke (blockage of blood vessels supplying food and oxygen to the brain) and hemorrhagic stroke (bleeding in the brain). Common symptoms of stroke include “sudden numbness or weakness, especially on one side of the body; sudden confusion or trouble speaking or understanding speech; sudden trouble seeing in one or both eyes; sudden trouble with walking, dizziness, or loss of balance or coordination; or sudden severe headache with no known cause.”44 According to the American Stroke Association, about 795,000 Americans each year suffer a new or recurrent stroke, with more than 129,000 deaths per year resulting from stroke.45
So, is there any evidence that fasting may reduce the risk of stroke or increase the odds of surviving a stroke? A number of studies suggest this is true. Perhaps the most important way fasting protects against stroke is the role it plays in preserving a healthy cardiovascular system and regulating blood pressure.
In a major clinical human trial conducted in the United States and published in the Proceedings of the National Academy of Science in 2011, doctors showed that people on a calorie reduction demonstrated reduction in well-known risk factors for ischemic stroke such as body fat, blood pressure, and serum lipid and lipoprotein levels. In that study researchers placed eighteen individuals on calorie reduction for about seven years, with a control group of another eighteen age-matched healthy individuals following a typical American diet. The researchers measured several biomarkers of cardiovascular disease, such as serum lipids and lipoproteins, fasting plasma glucose and insulin, blood pressure, high-sensitivity C-reactive protein, and body composition. At the end of seven years the fasting group showed normalized blood pressure. In addition, all the other factors for cardiovascular risks were higher in those who ate normal diets than for those who fasted.46
Simply put, this study showed convincingly that fasting reduces the risk of atherosclerosis, a condition closely linked to stroke. In fact, in another study researchers showed that BDNF and other neurotrophic factors were upregulated for fasting mice after experiencing ischemic stroke.47
So, does fasting protect in some ways against stroke or at least reduce the risk? Studies suggest it does. Human studies are still scarce, but we seem to have enough studies already accumulated in this area to take the outcomes seriously.