ESCAPING FROM PREDATORS An Integrative View of Escape Decisions (2015)
Part II Escape and refuge use: theory and findings for major taxonomic groups
IIb Escape decisions prior to pursuit
6 Fish and amphibians
Patricia A. Fleming and Philip W. Bateman
6.1 Introduction
Although at first glance there seem to be significant differences between fish and amphibians in their habitat and therefore predation risk, they share many similar biological features (particularly fish and larval amphibians, i.e., tadpoles) that govern their escape responses. Although anuran tadpoles fill a similar niche to fish, they have a different body form, with a globose body and compressed, tapered tail (Wassersug 1989). In terms of awareness of their environment, escape locomotion, and modifications of escape behavior, fish and amphibians share responses to the risk of potential predation (Wassersug 1989).
Teleost fish species make up about half of all vertebrate taxa (Volff 2005). This diversity of species means that there is a huge range in traits such as body size, color, presence of body armor, and swimming speed. There are also diverse lifestyles, from pelagic, schooling fish through to solitary benthic species. In addition to their biological differences, fish can also experience a range of environmental conditions (e.g., water turbidity, temperature, dissolved oxygen) that can make it more or less physically or physiologically difficult to detect an approaching threat or escape. All these factors can influence the ability of fish to escape detection and predation (Domenici 2010), and therefore influence their reliance on crypsis vs. swimming.
Perhaps stimulated by this marked diversity among fishes, many predictions of the economic escape model (EEM) have been tested in fish. Fish have also been tested under a wide range of conditions, reflecting their diversity of habitats and body forms.
Amphibians are considerably less diverse than fish in terms of species. However, their dramatic change in lifestyle between tadpoles and adults takes them, in most cases, from a three-dimensional water environment similar to that experienced by fish, to a relatively two-dimensional habitat with substantial gravitational effects, at least for some of the time. Visual acuity, visual range, auditory inputs, and the physical limitations of locomotion therefore change during the ontogeny of amphibians. Furthermore, this change can be accompanied by a change in predation risks. On land, adult amphibians are potential prey of many predators that they did not face as tadpoles. Cryptic coloration, immobility, and crouching serve as primary defenses by helping prevent detection, but active escape behavior is often needed, particularly in species without toxic skin secretions. There is a relative dearth of escape literature for amphibians, and what there is, is dominated by frogs. In the following chapter, we attempt to synthesize the escape behavior of both fish and, primarily, larval amphibians in the light of the predictions of the EEM (Ydenberg & Dill 1986).
6.2 The methodological challenges of working with fish and amphibians
6.2.1 How fish and amphibians perceive the world
Air and water have very different physical properties, and therefore one of the most significant differences between fish and adult amphibians is the medium in which they live. Light levels are attenuated rapidly in water, and therefore the effects of turbidity and depth have a marked effect on visual perception for these animals (see section 6.3.3.1). Visual cues trigger escape in response to looming and movement (e.g., Dill 1974a; Paglianti & Domenici 2006; Hettyey et al. 2012). Auditory cues are important for fish (reviewed by Popper & Fay 1993) and adult frogs (Cooper 2011 and references therein) and potentially for tadpoles (Hettyey et al. 2012). Olfactory cues (predator scents or cues of predated conspecifics) also modulate escape responses in fish (reviewed by Ferrari et al. 2010) and amphibians (Petranka et al. 1987; Semlitsch & Reyer 1992; Stauffer & Semlitsch 1993; Maag et al. 2012). Chemicals released from the skin in response to being attacked apparently act to alert conspecifics of imminent danger; adding the minced-up skins of conspecifics therefore alerts fish and amphibians to potential danger and increases their responses to predatory cues. Some fish (e.g., cartilaginous fishes) are sensitive to electrical stimuli. Because some of these senses exceed our own, and fish and amphibians have senses we lack, these signals often can be overlooked when studying the escape responses of fish and amphibians. We should, therefore, be aware of our inherent bias toward visual or auditory cues.
6.2.2 Neural control of escape behavior
Because each encounter with a predator is potentially lethal, there is strong selection on efficient and effective escape mechanisms. Fish and amphibians make interesting subjects for investigation, since their escape behavior has arguably been stripped back to the most basic responses. Fish and amphibians have some of the simplest vertebrate brains (they have no neocortex); despite this, there is evidence that fish can modify their escape behaviors based on learning and experience (Brown & Laland 2003; Kelley & Magurran 2003).
Immediate escape responses, e.g., the fast burst escape swimming of teleosts and the escape leap in frogs, have strong selection acting upon them, and consequently have become hard-wired as short reflex response neuronal loops in fish and amphibians that generate optimal behavioral responses; these initial reflexes are involuntary and nearly instantaneous “stereotypic” movements in response to stimulus (Eaton et al. 2001) (Box 6.1). Further aspects of escape (e.g., the distance moved and speed and trajectory of escape) are modified by the environmental context or predator type and approach (reviewed for fish by Domenici 2010), and therefore variability in these responses can be dynamically modified as predicted by the EEM (Ydenberg & Dill 1986; Walker et al. 2005; Domenici 2010; Marras et al. 2011; Chapter 8).
Box 6.1 “I’m getting out of here!” Short-burst escape responses in fish and amphibians
Both fish and amphibians show dramatic initial escape responses to threatening stimuli, the kinematics, performance, and physiology of which have been well studied (Domenici & Blake 1997; Eaton et al. 2001; Wakeling 2005; Walker et al. 2005; Domenici 2010). These brainstem escape networks can be stimulated by sensory input from sound, mechanical vibration, electrical field, or visual cues (Eaton et al. 2001).
The Mauthner cells are a single pair of giant, bilateral neurons (one for each half of the body) present in the hindbrains of fish and amphibians that mediate a very fast initial escape reflex, as well as participating in a larger parallel, brainstem escape network (Eaton et al. 2001). They have been described as “command neurons” (Eaton et al. 2001) which are envisioned as a type of neural decision-making cell that could trigger a complete behavioral act with little or no need for additional input from the command neuron.
In fish, the most extreme response to a threatening stimulus is the “fast start” escape response (Figure 6.1), which is a short burst (less than ~1 s) of high-energy swimming associated with a short latency and high acceleration and speed (Domenici & Blake 1997; Turesson et al. 2009). Two main types of fast-starts are recognized, C-starts and S-starts in which the fish is bent into a “C” or “S” shape at the end of the first contraction of the lateral musculature. The C-starts are generally associated with escape responses away from the threat, while the S-starts with prey capture where the fish moves toward prey.
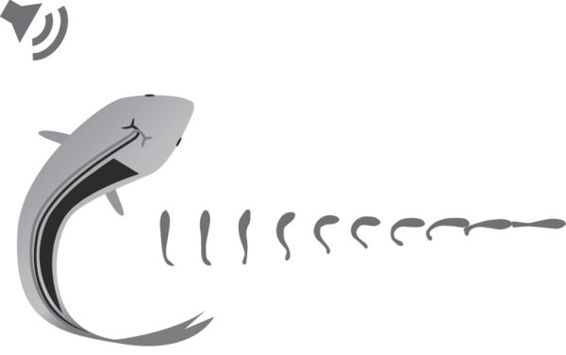
Figure 6.1
Illustration of the fast-start escape response in fish.
(Modified from Eaton et al. 2001)
Amphibians have a similarly fast escape response system. Tadpoles have a C-start, combined with an extremely flexible tail allowing rapid turning with little or no displacement of their center of mass (Wassersug 1989). In adult frogs, hard-wired stereotyped movement patterns are evident as an explosive long leap (Ingle & Hoff 1990) or diving response (Korn & Faber 2005). In an analogous situation as observed between C-start and S-start responses in fish, frogs also have separate post-tectal pathways stimulated for turning toward prey and turning away from threat (King & Comer 1996), and both short-term and long-term memories influence jump direction (Ingle & Hoff, 1990). These separate neural control mechanisms may account for why a number of studies reveal independent regulation between predation avoidance and effective foraging (e.g., Horat & Semlitsch 1994).
6.2.3 Logistical issues
One of the major difficulties in comparing these studies is that vastly different stimuli have been used to test the escape responses of different taxa (Figure 6.2). Most studies of small fish and tadpoles have been carried out in small tanks under captive conditions. Larger fish species have principally been examined in the field, e.g., tested for responses to approaching models of predators, humans (i.e., swimmers or wading), or responses to being passed over by a boat. Frog responses have all been tested in response to human approach on foot in the wild.
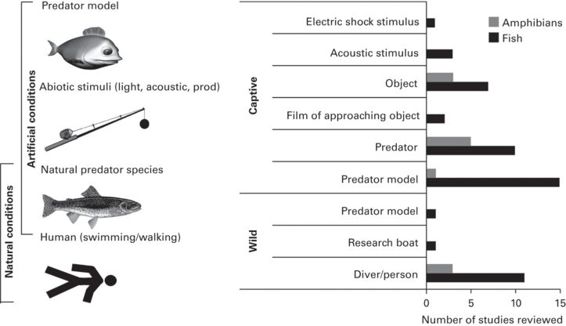
Figure 6.2
A wide range of stimuli have been used to initiate escape responses in fish and amphibians (frogs and tadpoles). The most common method for use in captive studies has been presenting prey with predator models; this has the advantage over real predators of being able to control the speed and type of approach. Under natural conditions, most studies have explored how fish and frogs respond to an approaching person, either swimming, wading or walking toward them.
While it is intuitive to human observers how many vertebrates react to approaching predators or disturbance (e.g., “alert” behavior, cessation of previous behavior, gaze direction, alarm calling), this is less clear for fish and amphibians. It is not obvious when these animals are “alert” to the predator’s presence, which means that we need to be very cautious in interpreting “monitoring” or “alert distance” in these animals.
There are also problems with measuring responses of individuals when they are part of a school (reviewed by Krause 1994). Instead, researchers have had to rely on measures such as “responsiveness” (the proportion of a group responding to a stimulus; Blaxter et al. 1981), the proportion of animals moving in a particular direction (Domenici & Batty 1997), or “response latency” (the interval of time between stimulus presentation and the first detectable movement of the fish; Domenici & Batty 1997). With some of these measures, it can be difficult to distinguish between prey responses to the approaching predator and responses to conspecifics. As fish group size increases, it appears that both speed and accuracy of decision-making increase when under predatory threat (Ward et al. 2011). Tendency of individuals to join shoals is presumed to be a trade-off between predation risk and foraging efficiency. In three-spined sticklebacks Gasterosteus aculeatus, bolder individuals tend to be at the front of shoals where both predation risk and foraging opportunities are greater (Ward et al. 2004). An individual animal’s antipredator behavior is therefore influenced by whether it is approached when alone or in a group of fish (Domenici & Batty 1997).
6.3 Predictions under the Economic Escape Model: 1. Increased risk of capture
Ydenberg and Dill (1986, 234) stated that “If other things are equal, the risk of death in a given encounter with a predator should increase with the approach velocity of the predator and the distance to effective cover; it should decrease with the attainable escape velocity of the prey.” Simply (and assuming that other things are equal), we predict that prey should therefore have longer flight initiation distance (FID), longer distance fled (DF), and faster escape speeds (“escape responses”) if they face increased risk of capture (Table 6.1). We discuss empirical support for these predictions below.
Table 6.1 Review of the literature for fish and amphibians, showing the predictions of the EEM in terms of flight initiation distance (FID), distance fled (DF), and escape speed (arrows: ↑ increase predicted, ↓ decrease predicted) and the number of studies we located that either supported these predictions (highlighted cells) or did not (non-highlighted cells).
|
Flight initiation distance (FID) |
Distance fled (FD) |
Escape speed |
||||||||||||
|
Prediction |
Decreases |
Same |
Increases |
Prediction |
Decreases |
Same |
Increases |
Prediction |
Decreases |
Same |
Increases |
|||
|
1. Increased risk of capture |
↑ |
↑ |
||||||||||||
|
if prey is slower |
↑ |
↑ |
||||||||||||
|
e.g., environment of prey |
O2 or CO2 level |
↑ |
2-4 |
↑ |
||||||||||
|
pollution |
↑ |
5,6 |
↑ |
|||||||||||
|
parasitism |
↑ |
|||||||||||||
|
osmotic shock |
↑ |
|||||||||||||
|
ammonia in water |
||||||||||||||
|
temperature (heat shock) |
↑ |
|||||||||||||
|
If prey is |
bright and colorful |
↑ |
↑ |
|||||||||||
|
distracted (e.g., foraging, fighting) |
↑ |
10-13 |
↑ |
|||||||||||
|
experienced (can identify predator) |
↑ |
14 |
14-18 |
↑↓ |
19 |
16 |
16,19,20 |
|||||||
|
is exposed to intensive fishing |
↑ |
21-27 |
↑ |
|||||||||||
|
environment interferes with prey senses or responses |
turbidity (decreased visibility) |
↑ |
28-30 |
↑ |
31 |
31 |
||||||||
|
greater distance to cover |
↑ |
32 |
25,32-34 |
↑ |
34 |
|||||||||
|
predator |
more predators |
↑ |
↑ |
|||||||||||
|
faster predator |
↑ |
28,35 |
36 |
↑ |
↑ |
28,36 |
||||||||
|
persistent predator |
↑ |
↑ |
||||||||||||
|
2. Increased cost of fleeing |
↓ |
↓ |
||||||||||||
|
prey |
loss of crypsis |
↓ |
↓ |
|||||||||||
|
body size (large fish - swimming less costly) |
↑ |
37 |
33,38-40 |
↑ |
||||||||||
|
injury that increases cost of fleeing |
↓ |
↓ |
||||||||||||
|
gravid |
↓ |
↓ |
||||||||||||
|
opportunity costs |
hungry (leaving food) |
↓ |
33 |
↓ |
||||||||||
|
3. Effectiveness of alternative defense tactics |
||||||||||||||
|
crypsis |
↓ |
41,42 |
↓ |
42,43 |
||||||||||
|
armor (e.g., spines, plates) |
↓ |
37 |
↓ |
44,45 |
44-46 |
|||||||||
|
poison (frogs only) |
||||||||||||||
|
4. Group size |
FID varies according to the fitness benefits attached to group membership of different sizes |
↑↓ |
37,47 |
48 |
30,49 |
|||||||||
References: 1. Allan et al. (2013); 2. Domenici et al. (2007); 3. Lefrancois & Domenici (2006); 4. Lefrançois et al. (2005); 5. Alvarez et al. (2006); 6. Krause et al. (2010); 7. Handeland et al. (1996); 8. McKenzie et al. (2009); 9. Webb & Zhang (1994); 10. Krause & Godin (1996); 11. Brick (1998); 12. Bohórquez-Herrera et al. (2013); 13. Jakobsson et al. (1995); 14. Healey & Reinhardt (1995); 15. Arai et al. (2007); 16. Dill (1974); 17. Malavasi et al. (2004); 18. D’Anna et al. (2012); 19. Ghalambor et al. (2004); 20. Langerhans et al. (2004); 21. Januchowski-Hartley et al. (2011); 22. Januchowski-Hartley et al. (2012); 23. Kulbicki (1998); 24. Feary et al. (2011); 25. Gotanda et al. (2009); 26. Januchowski-Hartley et al. (2013); 27. Cole (1994); 28. Meager et al. (2006); 29. Miner & Stein (1996); 30. Semeniuk & Dill (2005); 31. Hartman & Abrahams (2000); 32. McLean & Godin (1989); 33. Grant & Noakes (1987); 34. Dill (1990); 35. Fuiman (1993); 36. Dill (1974); 37. Abrahams (1995); 38. Webb (1981); 39. Paglianti & Domenici (2006); 40. Miller et al. (2011); 41. Radabaugh (1989); 42. Cooper et al. (2008); 43. Eterovick et al. (2010); 44. Andraso & Barron (1995); 45. Bergstrom (2002); 46. Andraso (1997); 47. Seghers (1981); 48. Godin & Morgan (1985); 49. Semeniuk & Dill (2006).
6.3.1 Prey attributes
6.3.1.1 When prey is slower
Prey locomotion has an effect on predator success. For example, a one standard deviation increase in fast-start performance in guppies Poecilia reticulata increases by two- to three-fold the odds of surviving a predation strike by pike cichlid Crenicichla alta (Walker et al. 2005). Predatory fish (Micropterus salmoides) are more likely to abort attacks and less likely to chase prey when their fish prey show higher acceleration performance (Webb 1986).
Escape speed in tadpoles is influenced by their body form (Dayton et al. 2005), as well as environment and previous experience. Rana dalmatina tadpoles reared with pursuit predators (sticklebacks) can swim faster (and have longer and deeper tail muscles) than conspecifics raised with ambush predators (dragonfly larvae) or no predators (Teplitsky et al. 2005). In a comparison across species, in addition to speed, evasiveness and habitat use also influence risk: Bufo bufo tadpoles are highly susceptible to predation by dragonfly larvae due to their constant slow movements, while Hyla arborea tadpoles, although slow generally, also showed high evasiveness, while R. dalmatina tadpoles were the least susceptible being benthic and immobile but capable of high bursts of speed (Chovanec 1992).
Larger fish may not be targeted by gape-limited predators (Januchowski-Hartley et al. 2011), and larger fish can also generally move faster than smaller fish (Videler 1993). We discuss the effects of body size on costs of fleeing in section 6.4.2. However, other factors may substantially affect swimming speed in fish. Water is 800 times denser than air and hence it is more difficult to displace; consequently, movement through water causes more turbulence and drag and requires more energy than moving through air (Denny 1993). Water also has lower oxygen levels than air (Denny 1993). Because escape speed is so important, the physiological state of prey is important in determining whether their escape is successful. Decreased oxygen levels, elevated CO2 and ammonia in water, pollution, parasitism, being injured or gravid, or subject to physiological shock (e.g., osmotic shock or heat shock) can all contribute to impaired escape in fish, generally due to impaired neural function and locomotion. Under the predictions of the EEM, this decreased mobility would increase risk of capture and should influence FID as fish respond with greater caution on account of their increased vulnerability; however, most empirical findings contradict these predictions. A number of studies have found no difference in FID of fish subject to low oxygen levels (Lefrancois & Domenici 2006; Domenici et al. 2007). Similarly, Lefrancois et al. (2005) reported no difference in escape speed; while Lefrancois and Domenici (2006) reported an increase in escape speed. Krause et al. (2010) report no effect of parasitism on FID. Similarly, Alvarez et al. (2006) and Krause et al. (2010) found no change in FID for fish exposed to pollution, while Alvarez et al. (2006) also found no effect on escape speed. Handeland et al. (1996) reported a decrease in escape distance due to osmotic shock, McKenzie et al. (2009) reported a decrease in escape speed as a result of ammonia in the water, while Webb and Zhang (1994) report a decrease in FID as a consequence of heat shock. Recent studies investigating the effects of exposure to increased CO2 levels in water demonstrate reduced responsiveness for prey damselfish Pomacentrus amboinensis, although their dottyback Pseudochromis fuscus predators also succumb to the effects of increased CO2 and suffer reduced capture success when similarly exposed (Allan et al. 2013).
Since alertness (i.e., visual, auditory etc. responses contribute to FID) and locomotion (escape speed and duration, escape trajectories) are our usual measures of escape responses, the effects of injury and other impairment are confounded with the measures that we are making. Additionally, the predictions of the EEM are clouded in regard to injuries. If risk increases due to injury or other impairment (e.g., survival of tadpoles with tails damaged in previous predatory encounters is reduced; Semlitsch 1990) then FID, DF, etc., should increase, but if the same condition increases the cost of fleeing, then it is likely to decrease responses. If it does both, then it is possible that there are ambiguous escape responses, and the animal may be more likely to resort to changes in habitat use.
6.3.1.2 When prey is bright and colorful
The polymorphic frog Oophaga granulifera can rely more on crypsis or aposematism, depending on variation in conspicuousness, and this variation is also reflected in escape behavior, with FID in response to a bird model being higher for more conspicuous red morphs than for cryptic green morphs or intermediate morphs (Willink et al. 2013). Despite the diversity of color patterns in fish, we found no studies that tested the effects of prey coloration on their escape responses. It may be that brightly colored fish tend to rely on alternative defenses (e.g., including spines and toxins; section 6.5).
6.3.1.3 When prey is distracted
Prey that are engaged in other activities may be exposed to increased risk due to diverted attention (Chan & Blumstein 2011). The foraging position of guppies influenced their vulnerability to predation since guppies foraging with their heads down were more vulnerable and more likely to be attacked by a cichlid predator. However, guppies foraging with their heads down showed shorter FIDs than animals that were foraging horizontally or those that were not foraging at all (Krause & Godin 1996). Non-foraging guppies reacted sooner, having longer FIDs, and horizontally foraging individuals reacted before downward-foraging individuals (Krause & Godin 1996). Bohórquez-Herrera et al. (2013) similarly showed silver-spotted sculpins Blepsias cirrhosus had reduced responsiveness during prey handling (compared with non-foraging or fish that were targeting prey).
As another form of “distraction,” golden dwarf cichlids Nannacara anomala engaged in fights demonstrate a decrease in FID (Jakobsson et al. 1995). In the presence of a predator model, fighting males changed their fighting behavior, showing more lateral display and tail beating (presumably behaviors that allow them to simultaneously monitor their environment) in preference over mouth wrestling (Brick 1998).
6.3.1.4 When prey has previous experience
The cognitive abilities of fish are more developed than most might assume (Laland et al. 2003). Previous experience with predators can influence subsequent behavior of fish: intensive catch and release of rainbow trout Oncorhyncus mykiss by anglers results in a rapid decline in catch rates within seven to ten days, suggesting that caught and released fish learnt to ignore hooks (Askey et al. 2006). Guppies learn to avoid trawl nets by escaping through a hole; a skill that was socially transmitted through observing previously trained conspecifics (Reader et al. 2003).
Consistent with the predictions of the EEM, as a result of experience with a predator, an increase in FID (Dill 1974b; Healey & Reinhardt 1995; D’Anna et al. 2004; Malavasi et al. 2004; Arai et al. 2007), DF (Ghalambor et al.2004), and escape speed (Dill 1974b; Ghalambor et al. 2004; Langerhans et al. 2004) have been recorded. Some interesting variations in escape response might suggest species differences as a consequence of previous experience. For example, Healey and Reinhardt (1995) reported changes in FID for experienced coho Oncorhynchus kisutch and chinook salmon O. tshawytscha over their predator-naïve responses: coho increased their FID (i.e., benefitted from experience and showed more flight behavior), but experienced chinook were more likely to stay immobile. These two species therefore show different tactics in response to the same predator, with coho using “a strategy based on rapid and early flight with, perhaps, a dependence on maneuverability and school cohesion to avoid capture” (Healey & Reinhardt 1995: 621), a strategy that may be more successful in the presence of safe refuge under natural conditions.
Consistent with the predictions of the EEM, a number of studies reveal that there is an increase in FID in response to high levels of fishing compared with marine reserves (Cole 1994; Kulbicki 1998; Gotanda et al. 2009; Feary et al. 2011; Januchowski-Hartley et al. 2011, 2012, 2013; Figure 6.3). This may reflect social learning among individuals that are able to escape, or alternatively, increased FID may simply tell us that we have removed all the less wary (i.e., more curious) individuals from the population (Sutter et al. 2012). Fish targeted by spear-fishing also alter their use of refuge as a result of exposure to fishing - at protected reefs, sea breams Diplodus sargus and D. vulgaris frequently swam into the closest shelters, whereas in fished reefs they mostly escaped in open water (Guidetti et al. 2008).
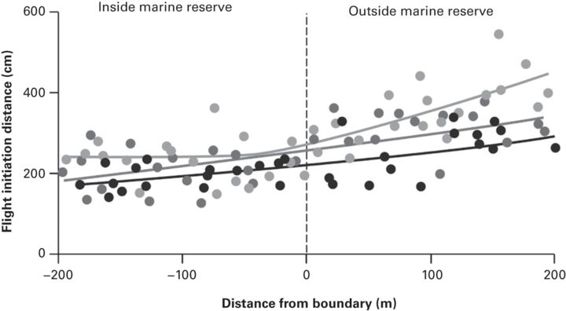
Figure 6.3
Relationship between FID for surgeon fishes (Acanthuridae) and distance from a marine reserve boundary. The different shaded dots represent data from three sites.
(Januchowski-Hartley et al. 2013)
Fish will often show predator inspection behavior, where a prey fish fixates on a predator, and slowly swims toward it (Pitcher et al. 1986). While inherently risky, this behavior may allow assessment of predator intent and dissuasion of predation, while also advertising fitness to potential mates (Sutter et al. 2012). However, this behavior may make fishes particularly vulnerable to spear fishers, bringing them closer to the fisher, and highlighting the fish as a target. Consequently, differences in responses by various fish families to fishing pressures may reflect differences in longevity, trophic level, availability of and differential use of refuges, or potentially group size (influencing social learning) (Januchowski-Hartley et al. 2011, 2013).
6.3.2 When the predator represents more risk
A faster predator will represent greater risk, and therefore prey should show increased escape responses (Table 6.1). There is empirical support of these predictions for fish, but we have found no data for amphibians in this respect. Dill (1974a) reported longer FID in zebra danios (Brachydanio rerio) in the presence of a faster approaching model of a predator, but others (Fuiman 1993; Meager et al. 2006) reported shorter FID in Clupea harengus and Gadus morhua, respectively. Interestingly, we found no published empirical tests of the effects of number of predators present or the persistence of predators on escape responses.
6.3.3 When the environment interferes with prey senses or responses
6.3.3.1 Turbidity (decreased visibility)
Conditions that make it harder to detect a predator (and therefore greater risk of the predator approaching while the prey is unaware) should elicit greater responsiveness in potential prey following predictions of the EEM. The problem is how to measure this change in responses. In an environment where visibility is reduced due to turbidity, the distance at which a predator is detected may be reduced below the optimal FID in clear conditions; therefore flight may be instantaneous on detection of a predator, but FID would be decreased. Numerous studies have shown that turbidity is correlated with decreased FID (Miner & Stein 1996; Semeniuk & Dill 2005; Meager et al. 2006). Prey therefore have less time to evade the predator in turbid water (Meager et al. 2006). There are, however, other ways of reacting to this increased risk. When in turbid water, fathead minnows Pimephales promelas display significantly fewer dashes in response to a visual predator stimulus, but fishes moved significantly further and faster in turbid waters (Hartman & Abrahams 2000).
6.3.3.2 Greater distance to cover
Predictions of the EEM suggest that animals show an increase in escape responses with greater distance to cover (Table 6.1). Data for fish indicate an increase in FID with greater distance to cover (Grant & Noakes 1987; McLean & Godin 1989; Dill 1990; Gotanda et al. 2009). Dill (1990) recorded no difference in fish escape speed with distance to cover.
6.4 Predictions under the Economic Escape Model: 2. increased cost of fleeing
If fleeing is costly, prey should show shorter FID (Ydenberg & Dill 1986: 237), and shorter DF (Table 6.1). It is possible that escape speed would also be slower. We discuss empirical support for these predictions below.
6.4.1 Loss of crypsis
As soon as an animal starts to move away from an approaching predator, it will forfeit any potential benefit of crypsis (Ydenberg & Dill 1986), and therefore cryptic animals may benefit from not responding immediately to the presence of a predator. Ydenberg and Dill (1986) also cover the effects of crypsis under “alternative strategies” (see section 6.5.1). Cryptic animals should only move away when the benefits of moving outweigh those of remaining stationary. Martín et al. (2006) reported decreased FID for green frogs in the absence of vegetation; the frogs presumably allowed an observer to approach closer, remained for long periods immobile and cryptic, due to their green or brown skin coloration.
6.4.2 Body size
The size of a fish may be correlated with measures of fitness such as reproductive value, which may in turn influence risk assessment. Warner (1998) found that female bluehead wrasse Thallosoma bifasciatum, which are iteroparous, are relatively risk averse, which may reflect residual reproductive value of individuals. Larger fish can, in general, move faster than smaller fish (Videler 1993). Importantly, however, the methods of swimming vary with body size in fish. In parrotfish, there is a link between body size and type of swimming locomotion (Miller et al. 2011). Larger fish are more likely to use less costly but relatively slower escape (paired fin swimming), whereas smaller fish use an energetically more costly, but relatively faster escape (body and caudal fin swimming) (Figure 6.4). Under the EEM, both decreased cost and slower swimming (in larger fish) would predict greater FID. Supporting these predictions, various studies report an increase in FID with body size (Webb 1981; Grant & Noakes 1987; Gotanda et al. 2009; Januchowski-Hartley et al. 2011; Miller et al. 2011)(but see Abrahams 1995; and Feary et al. 2011 who report no effect).
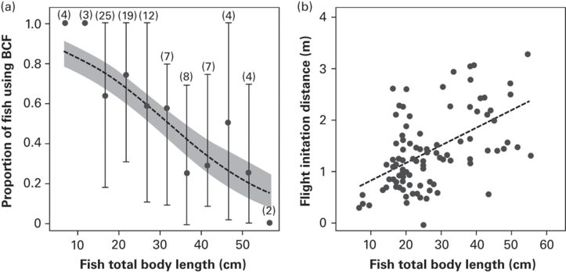
Figure 6.4
(a) In response to being approached by a snorkeler, small parrotfish are more likely to use body and caudal fin swimming (BCF), while large fish are more likely to swim away using slower paired fin swimming. (b) In the same study, there is a positive relationship between FID and body size across 95 parrotfish (three species combined).
(Miller et al. 2011)
In Southern Leopard frogs Lithobates sphenocephalus, larger frogs are more likely to hide in cover away from water than smaller ones (Bateman & Fleming 2014). When disturbed, they are more likely to resort to cover on land than smaller frogs, which retreat to water (Bateman & Fleming 2014). Ontogenetic changes in size can also influence escape tactics in tadpoles: Rana sylvatica tadpoles swim slowest when they are newly hatched and just prior to metamorphosis, which are also presumed to be the times when they are most susceptible to predation (Brown & Taylor 1995). At these times, they also show the highest propensity to engage in rapid turning behavior and ability to maneuver at sharper angles, which may compensate for decreased speed (Brown & Taylor 1995).
6.4.3 Opportunity costs
Escaping from a stimulus that is not threatening will ensure immediate safety, but incurs high opportunity costs (Chapters 2, 5). Therefore modulating responses is important, ensuring that the animal does not overreact to non-lethal stimuli. Consistent with the EEM, the presence of a food resource caused guppies (horizontally foraging ones only) to delay their flight compared to fish that were not given any food (Krause & Godin 1996). Similarly, unfed brook trout Salvelinus fontinalis decrease their FID in the presence of food (Grant & Noakes 1987). Feeding and antipredation behavior may be independently neurally controlled (Blanchard et al. 1991; Box 6.1); consequently, it may not be surprising that feeding activities can modulate antipredator responses.
An example of cost due to forfeiting opportunity in amphibians is illustrated in Box 6.2. Developing embryos will hatch prematurely in the presence of an immediate threat, forfeiting the opportunity of additional time developing in terrestrial nests away from aquatic predators.
Box 6.2 When you can’t wait around
Tadpole eggs are particularly vulnerable to predation during development and therefore frogs may preferentially lay eggs in vegetation above waterways to protect the eggs from aquatic predators. However, when approached by terrestrial predators, it is advantageous for these developing eggs to hatch rapidly (Figure 6.5), dropping to the water below and facing increased risk from aquatic predators as an underformed tadpole rather than face certain death (Warkentin, 1995). The eggs are sensitive to vibrations in their substrate, hatching up to 30% earlier than undisturbed nests (Warkentin, 2005).
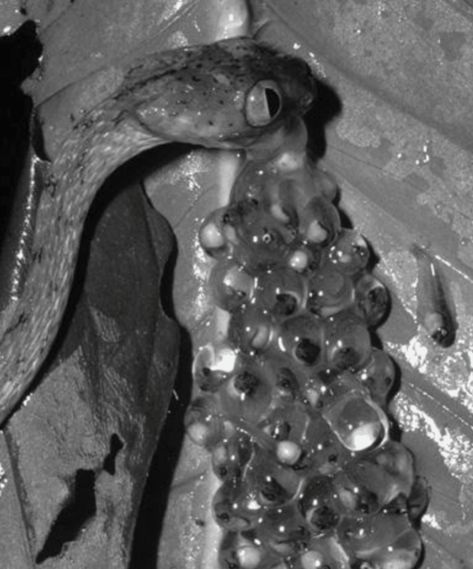
Figure 6.5
Chunk-headed snake Imantodes inornatus attacking an egg clutch of the treefrog Agalychnis callidryas.
(Photo by Karen Warkentin).
6.5 Predictions under the Economic Escape Model: 3. Effectiveness of alternative defense tactics
Effective alternative defenses would reduce “the cost of remaining at a given distance from an approaching predator” (Ydenberg & Dill 1986: 239). Therefore prey having such defenses should be less reliant on escape behavior and modify their responses accordingly.
6.5.1 Crypsis
Cryptic animals are predicted to be less responsive than more visible prey under the EEM. Several species of fish show counter-shading (Ruxton et al. 2004) or cryptic coloration (Donnelly & Dill 1984 and references therein). However, we found only one paper that specifically explored the influence of crypsis on escape behavior. Radabaugh (1989) showed that among male darters Etheostoma spp., those which exchanged cryptic colors for bright courtship colors became less likely to “freeze” and more likely to flee when approached by a simulated predatory threat.
To maintain the effect of crypsis, some fish have modified their escape behavior. Three species of nocturnal South American fish (the catfishes Tetranematichthys quadrifilis and Helogenes marmoratus and the knifefish Steatogenys duidae) are camouflaged as leaves: two species even lie on their sides, increasing their resemblance to fallen leaves (Sazima et al. 2006). When disturbed, they do not simply swim away; two species drift off like waterlogged leaves, while H. marmoratus moves up submerged root tangles, exposing its head and forebody, and looking like a leaf wedged in the roots.
When on substrates that enhance their crypsis, cryptic Bokermannohyla alvarengai tadpoles swim only short distances after disturbances and again become immobile (Eterovick et al. 2010). In adult frogs, immobility is crucial to maintaining crypsis, and is a major component of behavioral defense against predation. When approached by a human, over 90% of individuals belonging to five Craugastor species remained immobile until the predator reached them (Cooper et al. 2008).
6.5.2 Alternative defenses: poison
Because some frogs are poisonous, they may rely less on escape due to their aposomatic coloration and learned avoidance responses of predators. Cooper et al. (2009) examined the escape responses of poison dart frogs (Dendrobates auratus and Oophaga pumilio) and recorded low escape reactions: short FID and short DF - essentially the frogs got out of the way of the person’s approach and then stopped moving again. Although there are many venomous/poisonous fishes, it is surprising that there are no publications testing the predictions of the EEM in these animals.
6.5.3 Armor: spines and plates
Armor is an effective form of defense in fish (e.g., Lescak & von Hippel 2011). The influence of armor on escape behavior has been examined in fish, comparing the behavior of different species or different populations of the same species that have varying levels of armor (e.g., spines or bony plates). Consistent with the EEM, such studies report a decrease in FID for armored fish (Abrahams 1995), a decrease in escape distance (Andraso & Barron 1995; Bergstrom 2002), and a reduction in escape speed (Andraso & Barron 1995; Andraso 1997; Bergstrom 2002).
6.5.4 Retreat to safer sites
Retreating to cover is an important antipredator response in fish, frogs, and tadpoles. For example, several species of frog retreat to water from land when approached by humans (Martín et al. 2005, 2006; Cooper 2011; Bateman & Fleming 2014), which they use as cover (reviewed in Chapter 9).
6.6 Predictions under the Economic Escape Model: 4. Group size
Ydenberg and Dill (1986: 240) predicted that “if risk of predation or foraging efficiency varies with group size, then this will be reflected in flight distances,” but did not predict the direction of these changes because group size and membership have many facets that might affect FID, possibly in opposing ways. Because most predators target a single individual, the presence of group members decreases the risk of predation for each individual (risk dilution). The fitness benefits attached to group membership vary among group sizes, locations, food density, and predation regimes (Chapter 2).
Group membership can present an advantage of early warning (the “many eyes” hypothesis). Consequently, alert distance can be greater, and, since AD and FID are correlated, FID would be predicted to be greater for groups than solitary individuals: the whole group flees according to the most reactive individual. This has been tested with small groups of green frogs Rana perezi (Martín et al. 2006), but there was no difference in FID between solitary individuals and the first (most reactive) individual of small groups of up to four frogs. Similarly, Godin and Morgan (1985) found no difference in FID in response to a fish predator model between solitary and schooling banded killifish Fundulus diaphranus, and FID was statistically constant over a wide range of school sizes. However, other studies have found that escape responses vary with group size. Data for stingrays supports the early warning hypothesis (Semeniuk & Dill 2005, 2006) (Box 6.3). Interestingly, Abrahams (1995) showed that FID was shorter in grouped (n = 3) than solitary brook sticklebacks Culea incostans, suggesting that these animals were more sensitive to the effects of risk dilution than early warning. Because premature, or unwarranted responses to being approached can be costly, it is possible that the effects of group sizes on FID sometimes may only come into play when a minimal proportion of the group responds.
Box 6.3 Selective stingrays
Consistent with the early warning hypothesis, approaching cowtail stingrays Pastinachus sephen with a mock predator model, Semeniuk and Dill (2005) showed greater FID for the first cowtail in a group to flee compared with solitary cowtails. Cowtails will often settle next to reticulate whiprays Himantura uarnak, which have longer tails (Figure 6.6), show earlier responses than cowtails to a threat (approaching boat), and were most frequently the first to respond when in a mixed group (Semeniuk & Dill 2006). The whiprays have longer tails and may have an advantage in increased likelihood of predator detection via the mechanoreceptors found along the length of the tail. In 34 mixed-species groups, whiprays responded first 25 times (73%), although they made up only 49% of the membership of these groups. Whiprays could be preferred resting partners under conditions of poor visibility due to differences in predator-response capabilities between these two species. Cowtails also preferentially settle next to artificial ray shapes (unpainted marine plywood decoys) made with a longer tail, inspecting the tails before settling.

Figure 6.6
(a) Reticulate whiprays LHS have longer tails than (b) sympatric cowtail stingrays RHS.
Another consideration is the effect of group size on DF. If group cohesion is lost while fleeing, then perhaps DF should be shorter in a group. However, it appears that fish cohesion and reaction and response to predatory threat actually improve with increasing group size (Ward et al. 2011).
In Cuban tree frog tadpoles Osteopilus septentrionalis, fewer tadpoles swam away from a predatory stimulus from above when housed in higher density groups (Bateman & Fleming 2015). Among those that swim away, distance swum is shorter for tadpoles in smaller groups (Bateman & Fleming 2015).
6.7 Conclusions
As we reviewed the literature for this chapter, it became obvious that there is a massive literature on the escape responses of fishes, much of which is largely ignored in the literature of terrestrial taxa. Many key principles of the EEM have been tested in fish species, with varying results. Many studies do not support the simple predictions of the EEM (Table 6.1), either because the animals do not show measurable variation in their responses, or sometimes because they show responses that contradict the direction of our predictions. We have highlighted a few studies where careful examination of the responses of these animals can be explained through physiological effects of their environment, and it is clear that this needs to be considered carefully in terms of escape responses in fishes.
With regard to amphibians, it is surprising how little of the ecology of their antipredator escape has been explored. Ontogenetic change in antipredator responses as tadpoles metamorphose and move from water to land is an important aspect of the adaptation of frogs to life on land that reflects vulnerability to different suites of predators. Understanding these responses is likely to be an important tool for conservation management of frogs in these days of diminishing amphibian populations worldwide.
Finally, studies of escape behavior may provide information useful as a conservation tool for various taxa (Chapter 17). Escape behavior has been used as a measure of human impact on fish and frogs, and hence may prove useful for conservation of these animals (Blumstein & Fernández-Juricic 2010). For frogs, FID has been used to identify the effects of repeated exposure to human traffic for endangered Iberian frogs Rana iberica and to develop setback distances (Rodríguez-Prieto & Fernández-Juricic 2005). Escape behavior has also been widely used as a measure of environmental pollution on animals. For example, fish born of mothers exposed to methyl mercury demonstrate concentration-dependent effects on survival skills, including impairment of escape speeds(Alvarez et al. 2006). Rana blairi tadpoles exposed to sublethal levels of carbaryl (an insecticide) have reduced swimming performance and activity, which may result in increased predation and generate changes at the local population level (Bridges 1997). Recent observations that increased CO2 levels in water can impede escape responses (Allan et al.2013) rings warning bells for climate change predictions.
Escape behavior in fishes has been shown to be a powerful indicator of fishing intensity or even occasional poaching (Cole 1994; Gotanda et al. 2009; Feary et al. 2011; Januchowski-Hartley et al. 2011, 2013), and therefore could be used to gauge levels of compliance with no-take regulations of marine reserves. Escape measures are arguably more tractable for studies requiring high levels of replication than other survey methods (Figure 6.7), and are sensitive even to low levels of fishing (Feary et al. 2011; Januchowski-Hartley et al. 2012).
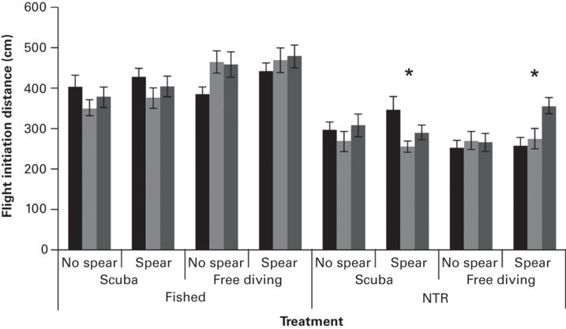
Figure 6.7
Flight initiation distance estimates (mean ± SE) for three observers (different shading) approaching fish in a fished or no-take marine reserve (NTR), using either scuba or free diving, and carrying a fishing spear or not. This study showed minor observer differences in estimates (asterisks) but the main effect was the difference between locations.
(Redrawn from Januchowski-Hartley et al. 2012)
References
Abrahams, M. V. (1995). The interaction between antipredator behaviour and antipredator morphology: Experiments with fathead minnows and brook sticklebacks. Canadian Journal of Zoology, 73, 2209-2215.
Allan, B. J. M., Domenici, P., Mccormick, M. I., Watson, S.-A. & Munday, P. L. (2013). Elevated CO2 affects predator-prey interactions through altered performance. PloS ONE, 8, e58520.
Alvarez, M. C., Murphy, C. A., Rose, K. A., Mccarthy, I. D. & Fuiman, L. A. (2006). Maternal body burdens of methylmercury impair survival skills of offspring in Atlantic croaker (Micropogonias undulatus). Aquatic Toxicology, 80, 329-337.
Andraso, G. M. (1997). A comparison of startle response in two morphs of the brook stickleback (Culaea inconstans): Further evidence for a trade-off between defensive morphology and swimming ability. Evolutionary Ecology, 11, 83-90.
Andraso, G. M. & Barron, J. N.(1995). Evidence for a trade-off between defensive morphology and startle-response performance in the brook stickleback (Culaea inconstans). Canadian Journal of Zoology, 73, 1147-1153.
Arai, T., Tominaga, O., Seikai, T. & Masuda, R. (2007). Observational learning improves predator avoidance in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles. Journal of Sea Research, 58, 59-64.
Askey, P. J., Richards, S. A., Post, J. R. & Parkinson, E. A. (2006). Linking angling catch rates and fish learning under catch-and-release regulations. North American Journal of Fisheries Management, 26, 1020-1029.
Bateman, P. W. & Fleming, P. A. (2014). Living on the edge: effects of body size, group density and microhabitat selection on escape behaviour of Southern Leopard Frogs (Lithobates sphenocephalus). Current Zoology, 60, 712-718.
Bateman, P. W. & Fleming, P. A. (2015). Body size and group size of Cuban tree frogs (Osteopilus Septentrionalis) tadpoles influence their escape behaviour. Acta Ethologica, doi: 10.1007/s10211-014-0201-9.
Bergstrom, C. (2002). Fast-start swimming performance and reduction in lateral plate number in threespine stickleback. Canadian Journal of Zoology, 80, 207-213.
Blanchard, R. J., Blanchard, D. C., Rodgers, J. & Weiss, S. M. (1991). The characterization and modelling of antipredator defensive behavior. Neuroscience & Biobehavioral Reviews, 14, 463-472.
Blaxter, J., Gray, J. & Denton, E. (1981). Sound and startle responses in herring shoals. Journal of the Marine Biology Association UK, 61, 851-870.
Blumstein, D. T. & Fernández-Juricic, E. (2010). A Primer on Conservation Behavior. Sunderland, MA: Sinauer Associates Inc.
Bohórquez-Herrera, J., Kawano, S. M. & Domenici, P.(2013). Foraging behavior delays mechanically-stimulated escape responses in fish. Integrative and Comparative Biology, 53, 780-786.
Brick, O. (1998). Fighting behaviour, vigilance and predation risk in the cichlid fish Nannacara anomala. Animal Behaviour, 56, 309-317.
Bridges, C. M. (1997). Tadpole swimming performance and activity affected by acute exposure to sublethal levels of carbaryl. Environmental Toxicology and Chemistry, 16, 1935-1939.
Brown, C. & Laland, K. N. (2003). Social learning in fishes: A review. Fish and Fisheries, 4, 280-288.
Brown, R. M. & Taylor, D. H. (1995). Compensatory escape mode trade-offs between swimming performance and maneuvering behavior through larval ontogeny of the wood frog, Rana sylvatica. Copeia, 1-7.
Chan, A. A. Y.-H. & Blumstein, D. T. (2011). Attention, noise, and implications for wildlife conservation and management. Applied Animal Behaviour Science, 131, 1-7.
Chovanec, A. (1992). The influence of tadpole swimming behaviour on predation by dragonfly nymphs. Amphibia-Reptilia, 13, 341-349.
Cole, R. (1994). Abundance, size structure, and diver-oriented behaviour of three large benthic carnivorous fishes in a marine reserve in northeastern New Zealand. Biological Conservation, 70, 93-99.
Cooper, W., E Jr. (2011). Escape strategy and vocalization during escape by American bullfrogs (Lithobates catesbeianus). Amphibia-Reptilia, 32, 213-221.
Cooper, W. E. Jr., Caldwell, J. P. & Vitt, L. J. (2008). Effective crypsis and its maintenance by immobility in Craugastor frogs. Copeia, 2008, 527-532.
Cooper, W. E. Jr., Caldwell, J. P. & Vitt, L. J. (2009). Risk assessment and withdrawal behavior by two species of aposematic poison frogs, Dendrobates auratus and Oophaga pumilio, on forest trails. Ethology, 115, 311-320.
D’anna, G., Giacalone, V. M., Badalamenti, F. & Pipitone, C.(2004). Releasing of hatchery-reared juveniles of the white seabream Diplodus sargus (L., 1758) in the Gulf of Castellammare artificial reef area (NW Sicily). Aquaculture, 233, 251-268.
D’anna, G., Giacalone, V. M., Fernández, T. V. et al. (2012). Effects of predator and shelter conditioning on hatchery-reared white seabream Diplodus sargus (L., 1758) released at sea. Aquaculture, 356-357, 91-97.
Dayton, G. H., Saenz, D., Baum, K. A., Langerhans, R. B. & Dewitt, T. J. (2005). Body shape, burst speed and escape behavior of larval anurans. Oikos, 111, 582-591.
Denny, M. W. (1993). Air and Water: The Biology and Physics of Life’s Media. Princeton University Press.
Dill, L. M. (1974a). The escape response of the zebra danio (Brachydanio rerio) I. The stimulus for escape. Animal Behaviour, 22, 711-722.
Dill, L. M. (1974b). The escape response of the zebra danio (Brachydanio rerio) II. The effect of experience. Animal Behaviour, 22, 723-730.
Dill, L. M. (1990). Distance-to-cover and the escape decisions of an African cichlid fish, Melanochromis chipokae. Environmental Biology of Fishes, 27, 147-152.
Domenici, P. (2010). Context-dependent variability in the components of fish escape response: Integrating locomotor performance and behavior. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 313, 59-79.
Domenici, P. & Batty, R. S.(1997). Escape behaviour of solitary herring (Clupea harengus) and comparisons with schooling individuals. Marine Biology, 128, 29-38.
Domenici, P. & Blake, R. (1997). The kinematics and performance of fish fast-start swimming. Journal of Experimental Biology, 200, 1165-1178.
Domenici, P., Lefrancois, C. & Shingles, A. (2007). Hypoxia and the antipredator behaviours of fishes. Philosophical Transactions of the Royal Society B: Biological Sciences, 362, 2105-2121.
Donnelly, W. A. & Dill, L. M. (1984). Evidence for crypsis in coho salmon, Oncorhynchus kisutch (Walbaum), parr: Substrate colour preference and achromatic reflectance. Journal of Fish Biology, 25, 183-195.
Eaton, R. C., Lee, R. K. K. & Foreman, M. B. (2001). The Mauthner cell and other identified neurons of the brainstem escape network of fish. Progress in Neurobiology, 63, 467-485.
Eterovick, P. C., Oliveira, F. F. R. & Tattersall, G. J. (2010). Threatened tadpoles of Bokermannohyla alvarengai (Anura: Hylidae) choose backgrounds that enhance crypsis potential. Biological Journal of the Linnean Society, 101, 437-446.
Feary, D. A., Cinner, J. E., Graham, N. A. J. & Januchowski-Hartley, F. A. (2011). Effects of customary marine closures on fish behavior, spear-fishing success, and underwater visual surveys. Conservation Biology, 25, 341-349.
Ferrari, M. C. O., Wisenden, B. D. & Chivers, D. P. (2010). Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Canadian Journal of Zoology, 88, 698-724.
Fuiman, L. A. (1993). Development of predator evasion in Atlantic herring, Clupea harengus L. Animal Behaviour, 45, 1101-1116.
Ghalambor, C. K., Reznick, D. N. & Walker, J. A. (2004). Constraints on adaptive evolution: the functional trade-off between reproduction and fast-start swimming performance in the Trinidadian guppy (Poecilia reticulata). The American Naturalist, 164, 38-50.
Godin, J.-G. J. & Morgan, M. J. (1985). Predator avoidance and school size in a cyprinodontid fish, the banded killifish (Fundulus diaphanus Lesueur). Behavioral Ecology and Sociobiology, 16, 105-110.
Gotanda, K. M., Turgeon, K. & Kramer, D. L. (2009). Body size and reserve protection affect flight initiation distance in parrot fishes. Behavioral Ecology and Sociobiology, 63, 1563-1572.
Grant, J. W. & Noakes, D. L. (1987). Escape behaviour and use of cover by young-of-the-year brook trout, Salvelinus fontinalis. Canadian Journal of Fisheries and Aquatic Sciences, 44, 1390-1396.
Guidetti, P., Vierucci, E. & Bussotti, S. (2008). Differences in escape response of fish in protected and fished Mediterranean rocky reefs. Journal of the Marine Biological Association of the UK, 88, 625-627.
Handeland, S. O., Järvi, T., Fernö, A. & Stefansson, S. O.(1996). Osmotic stress, antipredatory behaviour, and mortality of Atlantic salmon (Salmo salar) smolts. Canadian Journal of Fisheries and Aquatic Sciences, 53, 2673-2680.
Hartman, E. J. & Abrahams, M. V. (2000). Sensory compensation and the detection of predators: The interaction between chemical and visual information. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267, 571-575.
Healey, M. C. & Reinhardt, U. (1995). Predator avoidance in naive and experienced juvenile chinook and coho salmon. Canadian Journal of Fisheries and Aquatic Sciences, 52, 614-622.
Hettyey, A., Rölli, F., Thürlimann, N., Zürcher, A.-C. & Buskirk, J. V. (2012). Visual cues contribute to predator detection in anuran larvae. Biological Journal of the Linnean Society, 106, 820-827.
Horat, P. & Semlitsch, R. D. (1994). Effects of predation risk and hunger on the behaviour of two species of tadpoles. Behavioral Ecology and Sociobiology, 34, 393-401.
Ingle, D. J. & Hoff, K. (1990). Visually elicited evasive behavior in frogs. BioScience, 40, 284-291.
Jakobsson, S., Brick, O. & Kullberg, C. (1995). Escalated fighting behaviour incurs increased predation risk. Animal Behaviour, 49, 235-239.
Januchowski-Hartley, F. A., Feary, D., Morove, T. & Cinner, J. (2011). Fear of fishers: Human predation explains behavioral changes in coral reef fishes. PloS ONE, doi: 10.1371/journal.pone.0022761.
Januchowski-Hartley, F. A., Graham, N. A. J., Cinner, J. E. & Russ, G. R. (2013). Spillover of fish naïveté from marine reserves. Ecology Letters, 16, 191-197.
Januchowski-Hartley, F. A., Nash, K. L. & Lawton, R. J. (2012). Influence of spear guns, dive gear and observers on estimating fish flight initiation distance on coral reefs. Marine Ecology Progress Series, 469, 113.
Kelley, J. L. & Magurran, A. E. (2003). Learned predator recognition and antipredator responses in fishes. Fish and Fisheries, 4, 216-226.
King, J. R. & Comer, C. (1996). Visually elicited turning behavior in Rana pipiens: comparative organization and neural control of escape and prey capture. Journal of Comparative Physiology A, 178, 293-305.
Korn, H. & Faber, D. S. (2005). The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron, 47, 13-28.
Krause, J. (1994). Differential fitness returns in relation to spatial position in groups. Biological Reviews, 69, 187-206.
Krause, J. & Godin, J.-G. J. (1996). Influence of prey foraging posture on flight behavior and predation risk: Predators take advantage of unwary prey. Behavioral Ecology, 7, 264-271.
Krause, R. J., Grant, J. W., Mclaughlin, J. D. & Marcogliese, D. J. (2010). Do infections with parasites and exposure to pollution affect susceptibility to predation in johnny darters (Etheostoma nigrum)? Canadian Journal of Zoology, 88, 1218-1225.
Kulbicki, M. (1998). How the acquired behaviour of commercial reef fishes may influence the results obtained from visual censuses. Journal of Experimental Marine Biology and Ecology, 222, 11-30.
Laland, K. N., Brown, C. & Krause, J. (2003). Learning in fishes: From three-second memory to culture. Fish and Fisheries, 4, 199-202.
Langerhans, R. B., Layman, C. A., Shokrollahi, A. & Dewitt, T. J. (2004). Predator-driven phenotypic diversification in Gambusia affinis. Evolution, 58, 2305-2318.
Lefrancois, C. & Domenici, P. (2006). Locomotor kinematics and behaviour in the escape response of European sea bass, Dicentrarchus labrax L., exposed to hypoxia. Marine Biology, 149, 969-977.
Lefrançois, C., Shingles, A. & Domenici, P. (2005). The effect of hypoxia on locomotor performance and behaviour during escape in Liza aurata. Journal of Fish Biology, 67, 1711-1729.
Lescak, E. A. & Von Hippel, F. A. (2011). Selective predation of threespine stickleback by rainbow trout. Ecology of Freshwater Fish, 20, 308-314.
Maag, N., Gehrer, L. & Woodhams, D. C. (2012). Sink or swim: a test of tadpole behavioral responses to predator cues and potential alarm pheromones from skin secretions. Journal of Comparative Physiology A, 198, 841-846.
Malavasi, S., Georgalas, V., Lugli, M., Torricelli, P. & Mainardi, D. (2004). Differences in the pattern of antipredator behaviour between hatchery-reared and wild European sea bass juveniles. Journal of Fish Biology, 65, 143-155.
Marras, S., Killen, S. S., Claireaux, G., Domenici, P. & McKenzie, D. J. (2011). Behavioural and kinematic components of the fast-start escape response in fish: Individual variation and temporal repeatability. Journal of Experimental Biology, 214, 3102-3110.
Martín, J., Luque-Larena, J. J. & López, P. (2005). Factors affecting escape behavior of Iberian green frogs (Rana perezi). Canadian Journal of Zoology, 83, 1189-1194.
Martín, J., Luque-Larena, J. J. & López, P. (2006). Collective detection in escape responses of temporary groups of Iberian green frogs. Behavioral Ecology, 17, 222-226.
McKenzie, D. J., Shingles, A., Claireaux, G. & Domenici, P. (2009). Sublethal concentrations of ammonia impair performance of the teleost fast-start escape response. Physiological and Biochemical Zoology, 82, 353-362.
McLean, E. B. & Godin, J.-G. J. (1989). Distance to cover and fleeing from predators in fish with different amounts of defensive armour. Oikos, 281-290.
Meager, J. J., Domenici, P., Shingles, A. & Utne-Palm, A. C. (2006). Escape responses in juvenile Atlantic cod Gadus morhua L.: The effects of turbidity and predator speed. Journal of Experimental Biology, 209, 4174-4184.
Miller, B. M., Mcdonnell, L. H., Sanders, D. J. et al. (2011). Locomotor compensation in the sea: Body size affects escape gait in parrotfish. Animal Behaviour, 82, 1109-1116.
Miner, J. G. & Stein, R. A. (1996). Detection of predators and habitat choice by small bluegills: Effects of turbidity and alternative prey. Transactions of the American Fisheries Society, 125, 97-103.
Paglianti, A. & Domenici, P. (2006). The effect of size on the timing of visually mediated escape behaviour in staghorn sculpin Leptocottus armatus. Journal of Fish Biology, 68, 1177-1191.
Petranka, J. W., Kats, L. B. & Sih, A. (1987). Predator-prey interactions among fish and larval amphibians: Use of chemical cues to detect predatory fish. Animal Behaviour, 35, 420-425.
Pitcher, T. J., Green, D. A. & Magurran, A. E. (1986). Dicing with death: predator inspection behaviour in minnow shoals. Journal of Fish Biology, 28, 439-448.
Popper, A. N. & Fay, R. R. (1993). Sound detection and processing by fish: critical review and major research questions (Part 1 of 2). Brain, Behavior and Evolution, 41, 14-25.
Radabaugh, D. (1989). Seasonal colour changes and shifting antipredator tactics in darters. Journal of Fish Biology, 34, 679-685.
Reader, S. M., Kendal, J. R. & Laland, K. N. (2003). Social learning of foraging sites and escape routes in wild Trinidadian guppies. Animal Behaviour, 66, 729-739.
Rodríguez-Prieto, I. & Fernández-Juricic, E.(2005). Effects of direct human disturbance on the endemic Iberian frog Rana iberica at individual and population levels. Biological Conservation, 123, 1-9.
Ruxton, G. D., Speed, M. P. & Kelly, D. J. (2004). What, if anything, is the adaptive function of countershading? Animal Behaviour, 68, 445-451.
Sazima, I., Carvalho, L. N., Mendonça, F. P. & Zuanon, J.(2006). Fallen leaves on the water-bed: Diurnal camouflage of three night active fish species in an Amazonian streamlet. Neotropical Ichthyology, 4, 119-122.
Seghers, B. H. (1981). Facultative schooling behavior in the spottail shiner (Notropis hudsonius): Possible costs and benefits. Environmental Biology of Fishes, 6, 21-24.
Semeniuk, C. A. D. & Dill, L. M. (2005). Cost/benefit analysis of group and solitary resting in the cowtail stingray, Pastinachus sephen. Behavioral Ecology, 16, 417-426.
Semeniuk, C. A. D. & Dill, L. M. (2006). Anti-predator benefits of mixed-species groups of cowtail stingrays (Pastinachus sephen) and whiprays (Himantura uarnak) at rest. Ethology, 112, 33-43.
Semlitsch, R. D. (1990). Effects of body size, sibship, and tail injury on the susceptibility of tadpoles to dragonfly predation. Canadian Journal of Zoology, 68, 1027-1030.
Semlitsch, R. D. & Reyer, H.-U. (1992). Modification of anti-predator behaviour in tadpoles by environmental conditioning. Journal of Animal Ecology, 353-360.
Stauffer, H.-P. & Semlitsch, R. D. (1993). Effects of visual, chemical and tactile cues of fish on the behavioural responses of tadpoles. Animal Behaviour, 46, 355-364.
Sutter, D. A. H., Suski, C. D., Philipp, D. P. et al. (2012). Recreational fishing selectively captures individuals with the highest fitness potential. Proceedings of the National Academy of Sciences, 109, 20960-20965.
Teplitsky, C., Plénet, S., Léna, J. P. et al. (2005). Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. Journal of Evolutionary Biology, 18, 180-190.
Turesson, H. K., Satta, A. & Domenici, P. (2009). Preparing for escape: Anti-predator posture and fast-start performance in gobies. Journal of Experimental Biology, 212, 2925-2933.
Videler, J. J. (1993). Fish Swimming. London: Chapman & Hall.
Volff, J. N. (2005). Genome evolution and biodiversity in teleost fish. Heredity, 94, 280-294.
Wakeling, J. M. (2005). Fast-start mechanics. Fish Physiology, 23, 333-368.
Walker, J. A., Ghalambor, C. K., Griset, O. L., Mckenney, D. & Reznick, D. N. (2005). Do faster starts increase the probability of evading predators? Functional Ecology, 19, 808-815.
Ward, A. J., Herbert-Read, J. E., Sumpter, D. J. & Krause, J. (2011). Fast and accurate decisions through collective vigilance in fish shoals. Proceedings of the National Academy of Sciences, 108, 2312-2315.
Ward, A. J. W., Thomas, P., Hart, P. J. & Krause, J. (2004). Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behavioral Ecology and Sociobiology, 55, 561-568.
Warkentin, K. M. (1995). Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proceedings of the National Academy of Sciences, 92, 3507-3510.
Warkentin, K. M. (2005). How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Animal Behaviour, 70, 59-71.
Warner, R. R. (1998). The role of extreme iteroparity and risk avoidance in the evolution of mating systems. Journal of Fish Biology, 53, 82-93.
Wassersug, R. J. (1989). Locomotion in amphibian larvae (or “Why aren’t tadpoles built like fishes?”). American Zoologist, 29, 65-84.
Webb, P. W. (1981). Responses of northern anchovy, Engraulis mordax, larvae to predation by a biting planktivore, Amphiprion percula. Fishery Bulletin, 79.
Webb, P. W. (1986). Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides). Canadian Journal of Fisheries and Aquatic Sciences, 43, 763-771.
Webb, P. W. & Zhang, H. (1994). The relationship between responsiveness and elusiveness of heat-shocked goldfish (Carassius auratus) to attacks by rainbow trout (Oncorhynchus mykiss). Canadian Journal of Zoology, 72, 423-426.
Willink, B., Brenes-Mora, E., Bolaños, F. & Pröhl, H. (2013). Not everything is black and white: Color and behavioral variation reveal a continuum between cryptic and aposematic strategies in a polymorphic poison frog. Evolution, 67, 2783-2794.
Ydenberg, R. C. & Dill, L. M. (1986). The economics of fleeing from predators. Advances in the Study of Behavior, 16, 229-249.