Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality - Manjit Kumar (2009)
Part I. THE QUANTUM
Chapter 4. THE QUANTUM ATOM
Slagelse, Denmark, Thursday, 1 August 1912. The cobbled streets of the small, picturesque town some 50 miles south-west of Copenhagen were decked out in flags. Yet it was not in the beautiful medieval church, but in the civic hall that Niels Bohr and Margrethe Nørland were married in a two-minute ceremony conducted by the chief of police. The mayor was away on holiday, Harald was best man, and only close family were present. Like his parents before him, Bohr did not want a religious ceremony. He had stopped believing in God as a teenager, when he had confessed to his father: 'I cannot understand how I could be so taken in by all this; it means nothing whatsoever to me.'1 Had he lived, Christian Bohr would have approved when, a few months before the wedding, his son formally resigned from the Lutheran Church.
Originally intending to spend their honeymoon in Norway, the couple were forced to change their plans as Bohr failed to finish a paper on alpha particles in time. Instead the newlyweds travelled to Cambridge for a two-week stay during their month-long honeymoon.2 In between visits to old friends and showing Margrethe around Cambridge, Bohr completed his paper. It was a joint effort. Niels dictated, always struggling for the right word to make his meaning clear, while Margrethe corrected and improved his English. They worked so well together that for the next few years Margrethe effectively became his secretary.
Bohr disliked writing and avoided doing so whenever he could. He was able to complete his doctoral thesis only by dictating it to his mother. 'You mustn't help Niels so much, you must let him learn to write himself', his father had urged, to no avail.3 When he did put pen to paper, Bohr wrote slowly and in an almost indecipherable scrawl. 'First and foremost,' recalled a colleague, 'he found it difficult to think and write at the same time.'4 He needed to talk, to think aloud as he developed his ideas. He thought best while on the move, usually circling a table. Later, an assistant, or anyone he could find for the task, would sit with pen poised as he paced about dictating in one language or other. Rarely satisfied with the composition of a paper or lecture, Bohr would 'rewrite' it up to a dozen times. The end result of this excessive search for precision and clarity was often to lead the reader into a forest where it was difficult to see the wood for the trees.
With the manuscript finally completed and safely packed away, Niels and Margrethe boarded the train to Manchester. On meeting his bride, Ernest and Mary Rutherford knew that the young Dane had been lucky enough to find the right woman. The marriage indeed proved to be a long and happy one that was strong enough to endure the death of two of their six sons. Rutherford was so taken with Margrethe that for once there was little talk of physics. But he made time to read Bohr's paper and promised to send it to the Philosophical Magazine with his endorsement.5 Relieved and happy, a few days later the Bohrs travelled to Scotland to enjoy the remainder of their honeymoon.
Returning to Copenhagen at the beginning of September, they moved into a small house in the prosperous coastal suburb of Hellerup. In a country with only one university, physics posts rarely became vacant.6 Just before his wedding day, Bohr had accepted a job as a teaching assistant at the L![]() reanstalt, the Technical College. Each morning, Bohr would cycle to his new office. 'He would come into the yard, pushing his bicycle, faster than anybody else', recalled a colleague later.7 'He was an incessant worker and seemed always to be in a hurry.' The relaxed, pipe-smoking elder statesman of physics lay in the future.
reanstalt, the Technical College. Each morning, Bohr would cycle to his new office. 'He would come into the yard, pushing his bicycle, faster than anybody else', recalled a colleague later.7 'He was an incessant worker and seemed always to be in a hurry.' The relaxed, pipe-smoking elder statesman of physics lay in the future.
Bohr also began teaching thermodynamics as a privatdozent at the university. Like Einstein, he found preparing a lecture course arduous. Nevertheless, at least one student appreciated the effort and thanked Bohr for 'the clarity and conciseness' with which he had 'arranged the difficult material' and 'the good style' with which it had been delivered.8 But teaching combined with his duties as an assistant left him precious little time to tackle the problems besetting Rutherford's atom. Progress was painfully slow for a young man in a hurry. He had hoped that a report written for Rutherford while still in Manchester on his nascent ideas about atomic structure, later dubbed the 'Rutherford Memorandum', would serve as the basis of a paper ready for publication soon after his honeymoon.9 It was not to be.
'You see,' Bohr said 50 years later in one of the last interviews he gave, 'I'm sorry because most of that was wrong.'10 However, he had identified the key problem: the instability of Rutherford's atom. Maxwell's theory of electromagnetism predicted that an electron circling the nucleus should continuously emit radiation. This incessant leaking of energy sends the electron spiralling into the nucleus as its orbit rapidly decays. Radiative instability was such a well known failing that Bohr did not even mention it in his Memorandum. What really concerned him was the mechanical instability that plagued Rutherford's atom.
Beyond assuming that electrons revolved around the nucleus in the manner of planets around the sun, Rutherford had said nothing about their possible arrangement. A ring of negatively-charged electrons circling the nucleus was known to be unstable due to the repulsive forces the electrons exert on each other because they have the same charge. Nor could the electrons be stationary; since opposite charges attract, the electrons would be dragged towards the positively-charged core. It was a fact that Bohr recognised in the opening sentence of his memo: 'In such an atom there can be no equilibrium [con]figuration without the motion of electrons.'11 The problems that the young Dane had to overcome were mounting up. The electrons could not form a ring, they could not be stationary, and they could not orbit the nucleus. Lastly, with a tiny, point-like nucleus at its heart, there was no way in Rutherford's model to fix the radius of an atom.
Whereas others had interpreted these problems of instability as damning evidence against Rutherford's nuclear atom, for Bohr they signalled the limitations of the underlying physics that predicted its demise. His identification of radioactivity as a 'nuclear' and not an 'atomic' phenomenon, his pioneering work on radioelements, what Soddy later called isotopes, and on nuclear charge convinced Bohr that Rutherford's atom was indeed stable. Although it could not bear the weight of established physics, it did not suffer the predicted collapse. The question that Bohr had to answer was: why not?
Since the physics of Newton and Maxwell had been impeccably applied and forecast electrons crashing into the nucleus, Bohr accepted that the 'question of stability must therefore be treated from a different point of view'.12 He understood that to save Rutherford's atom would require a 'radical change', and he turned to the quantum discovered by a reluctant Planck and championed by Einstein.13 The fact that in the interaction between radiation and matter, energy was absorbed and emitted in packets of varying size rather than continuously, was something beyond the realm of time-honoured 'classical' physics. Even though like almost everyone else he did not believe in Einstein's light-quanta, it was clear to Bohr that the atom 'was in some way regulated by the quantum'.14 But in September 1912 he had no idea how.
All his life, Bohr loved to read detective stories. Like any good private eye, he looked for clues at the crime scene. The first were the predictions of instability. Certain that Rutherford's atom was stable, Bohr came up with an idea that proved crucial to his ongoing investigation: the concept of stationary states. Planck had constructed his blackbody formula to explain the available experimental data. Only then did he attempt to derive his equation and in the process stumbled across the quantum. Bohr adopted a similar strategy. He would begin by rebuilding Rutherford's atomic model so that electrons did not radiate energy as they orbited the nucleus. Only later would he try to justify what he had done.
Classical physics placed no restrictions on an electron's orbit inside an atom. But Bohr did. Like an architect designing a building to the strict requirements of a client, he restricted electrons to certain 'special' orbits in which they could not continuously emit radiation and spiral into the nucleus. It was a stroke of genius. Bohr believed that certain laws of physics were not valid in the atomic world and so he 'quantised' electron orbits. Just as Planck had quantised the absorption and emission of energy by his imaginary oscillators so as to derive his blackbody equation, Bohr abandoned the accepted notion that an electron could orbit an atomic nucleus at any given distance. An electron, he argued, could occupy only a few select orbits, the 'stationary states', out of all the possible orbits allowed by classical physics.
It was a condition that Bohr was perfectly entitled to impose as a theorist trying to piece together a viable working atomic model. It was a radical proposal, and for the moment all he had was an unconvincing circular argument that contradicted established physics - electrons occupied special orbits in which they did not radiate energy; electrons did not radiate energy because they occupied special orbits. Unless he could offer a real physical explanation for his stationary states, the permissible electron orbits, they would be dismissed as nothing more than theoretical scaffolding erected to hold up a discredited atomic structure.
'I hope to be able to finish the paper in a few weeks,' Bohr wrote to Rutherford at the beginning of November.15 Reading the letter and sensing Bohr's mounting anxiety, Rutherford replied that there was no reason 'to feel pressed to publish in a hurry' since it was unlikely anyone else was working along the same lines.16 Bohr was unconvinced as the weeks passed without success. If others were not already actively engaged in trying to solve the mystery of the atom, then it was only a matter of time. Struggling to make headway, in December he asked for and was granted a few months' sabbatical by Knudsen. Together with Margrethe, Bohr found a secluded cottage in the countryside as he set about searching for more atomic clues. Just before Christmas he found one in the work of John Nicholson. At first he feared the worst, but he soon realised that the Englishman was not the competitor he dreaded.
Bohr had met Nicholson during his abortive stay in Cambridge, and had not been overly impressed. Only a few years older at 31, Nicholson had since been appointed professor of mathematics at King's College, University of London. He had also been busy building an atomic model of his own. He believed that the different elements were actually made up of various combinations of four 'primary atoms'. Each of these 'primary atoms' consisted of a nucleus surrounded by a different number of electrons that formed a rotating ring. Though, as Rutherford said, Nicholson had made an 'awful hash' of the atom, Bohr had found his second clue. It was the physical explanation of the stationary states, the reason why electrons could occupy only certain orbits around the nucleus.
An object moving in a straight line has momentum. It is nothing more than the object's mass times its velocity. An object moving in a circle possesses a property called 'angular momentum'. An electron moving in a circular orbit has an angular momentum, labelled L, that is just the mass of the electron multiplied by its velocity multiplied by the radius of its orbit, or simply L=mvr. There were no limits in classical physics on the angular momentum of an electron or any other object moving in a circle.
When Bohr read Nicholson's paper, he found his former Cambridge colleague arguing that the angular momentum of a ring of electrons could change only by multiples of h/2![]() , where h is Planck's constant and
, where h is Planck's constant and ![]() (pi) is the well-known numerical constant from mathematics, 3.14….17 Nicholson showed that the angular momentum of a rotating electron ring could only be h/2
(pi) is the well-known numerical constant from mathematics, 3.14….17 Nicholson showed that the angular momentum of a rotating electron ring could only be h/2![]() or 2(h/2
or 2(h/2![]() ) or 3(h/2
) or 3(h/2![]() ) or 4(h/2
) or 4(h/2![]() ) … all the way to n(h/2
) … all the way to n(h/2![]() ) where n is an integer, a whole number. For Bohr it was the missing clue that underpinned his stationary states. Only those orbits were permitted in which the angular momentum of the electron was an integer n multiplied by h and then divided by 2
) where n is an integer, a whole number. For Bohr it was the missing clue that underpinned his stationary states. Only those orbits were permitted in which the angular momentum of the electron was an integer n multiplied by h and then divided by 2![]() . Letting n=1, 2, 3 and so on generated the stationary states of the atom in which an electron did not emit radiation and could therefore orbit the nucleus indefinitely. All other orbits, the non-stationary states, were forbidden. Inside an atom, angular momentum was quantised. It could only have the values L=nh/2
. Letting n=1, 2, 3 and so on generated the stationary states of the atom in which an electron did not emit radiation and could therefore orbit the nucleus indefinitely. All other orbits, the non-stationary states, were forbidden. Inside an atom, angular momentum was quantised. It could only have the values L=nh/2![]() and no others.
and no others.
Just as a person on a ladder can stand only on its steps and nowhere in between, because electron orbits are quantised, so are the energies that an electron can possess inside an atom. For hydrogen, Bohr was able to use classical physics to calculate its single electron's energy in each orbit. The set of allowed orbits and their associated electron energies are the quantum states of the atom, its energy levels En. The bottom rung of this atomic energy ladder is n=1, when the electron is in the first orbit, the lowest-energy quantum state. Bohr's model predicted that the lowest energy level, E1, called the 'ground state', for the hydrogen atom would be -13.6eV, where an electron volt (eV) is the unit of measurement adopted for energy on the atomic scale and the minus sign indicates that the electron is bound to the nucleus.18 If the electron occupies any other orbit but n=1, then the atom is said to be in an 'excited state'. Later called the principal quantum number, n is always an integer, a whole number, which designates the series of stationary states that an electron can occupy and the corresponding set of energy levels, En, of the atom.
Bohr calculated the values of the energy levels of the hydrogen atom and found that the energy of each level was equal to the energy of the ground state divided by n2, (E1/n2)eV. Thus, the energy value for n=2, the first excited state, is -13.6/4 = -3.40eV. The radius of the first electron orbit, n=1, determines the size of the hydrogen atom in the ground state. From his model, Bohr calculated it as 5.3 nanometres (nm), where a nanometre is a billionth of a metre - in close agreement with the best experimental estimates of the day. He discovered that the radius of the other allowed orbits increased by a factor of n2: when n=1, the radius is r; when n=2, then the radius is 4r; when n=3, the radius is 9r and so on.
'I hope very soon to be able to send you my paper on the atoms,' Bohr wrote to Rutherford on 31 January 1913, 'it has taken far more time than I had thought; I think, however, that I have made some progress in it in the latest time.'19 He had stabilised the nuclear atom by quantising the angular momentum of the orbiting electrons, and thereby explained why
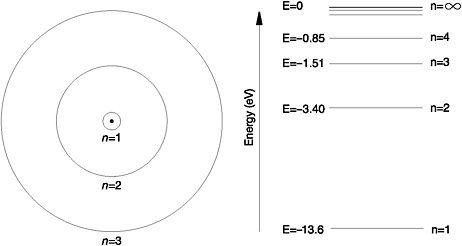
Figure 6: Some of the stationary states and the corresponding energy levels of the hydrogen atom (not drawn to scale)
they could occupy only a certain number, the stationary states, of all possible orbits. Within days of writing to Rutherford, Bohr came across the third and final clue that allowed him to complete the construction of his quantum atomic model.
Hans Hansen, a year younger and a friend of Bohr's from their student days in Copenhagen, had just returned to the Danish capital after completing his studies in Göttingen. When they met, Bohr told him about his latest ideas on atomic structure. Having conducted research in Germany in spectroscopy, the study of the absorption and emission of radiation by atoms and molecules, Hansen asked Bohr if his work shed any light on the production of spectral lines. It had long been known that the appearance of a naked flame changed colour depending upon which metal was being vaporised: bright yellow with sodium, deep red with lithium, and violet with potassium. In the nineteenth century it had been discovered that each element produced a unique set of spectral lines, spikes in the spectrum of light. The number, spacing and wavelengths of the spectral lines produced by the atoms of any given element are unique, a fingerprint of light that can be used to identify it.
Spectra appeared far too complicated, given the enormous variety of patterns displayed by the spectral lines of the different elements, for anyone to seriously believe that they could be the key to unlocking the inner workings of the atom. The beautiful array of colours on a butterfly's wing were all very interesting, Bohr said later, 'but nobody thought that one could get the basis of biology from the colouring of the wing of a butterfly'.20 There was obviously a link between an atom and its spectral lines, but at the beginning of February 1913 Bohr had no inkling what it could be. Hansen suggested that he take a look at Balmer's formula for the spectral lines of hydrogen. As far as Bohr could remember, he had never heard of it. More likely he had simply forgotten it. Hanson outlined the formula and pointed out that no one knew why it worked.
Johann Balmer was a Swiss mathematics teacher at a girls' school in Basel and a part-time lecturer at the local university. Knowing that he was interested in numerology, a colleague told Balmer about the four spectral lines of hydrogen after he had complained about having nothing interesting to do. Intrigued, he set out to find a mathematical relationship between the lines where none appeared to exist. The Swedish physicist, Anders Ångström, had in the 1850s measured the wavelengths of the four lines in the red, green, blue and violet regions of the visible spectrum of hydrogen with remarkable accuracy. Labelling them alpha, beta, gamma and delta respectively, he found their wavelengths to be: 656.210, 486.074, 434.01 and 410.12nm.21 In June 1884, as he approached 60, Balmer found a formula that reproduced the wavelengths (![]() ) of the four spectral lines:
) of the four spectral lines: ![]() = b[m2/(m2-n2)] in which m and n are integers and b is a constant, a number determined by experiment as 364.56nm.
= b[m2/(m2-n2)] in which m and n are integers and b is a constant, a number determined by experiment as 364.56nm.
Balmer discovered that if n was fixed as 2 but m set equal to 3, 4, 5 or 6, then his formula gave an almost exact match for each of the four wavelengths in turn. For example, when n=2 and m=3 is plugged into the formula, it gives the wavelength of the red alpha line. However, Balmer did more than just generate the four known spectral lines of hydrogen, later named the Balmer series in his honour. He predicted the existence of a fifth line when n=2 but m=7, unaware that Ångström, whose work was published in Swedish, had already discovered and measured its wavelength. The two values, experimental and theoretical, were in near-perfect agreement.
Had Ångström lived (he died in 1874 aged 59), he would have been astounded by Balmer's use of his formula to predict the existence of other series of spectral lines for the hydrogen atom in the infrared and ultraviolet regions by simply setting n to 1, 3, 4 and 5 while letting m cycle through different numbers, as he had done with n set at 2 to generate the four original lines. For example, with n=3 and m=4 or 5 or 6 or 7…, Balmer predicted the series of lines in the infrared that were discovered in 1908 by Friedrich Paschen. Each of the series forecast by Balmer was later discovered, but no one had been able to explain what lay behind the success of his formula. What physical mechanism did the formula, arrived at through a process of trial and error, symbolise?
'As soon as I saw Balmer's formula,' Bohr said later, 'the whole thing was immediately clear to me.'22 It was electrons jumping between different allowed orbits that produced the spectral lines emitted by an atom. If a hydrogen atom in the ground state, n=1, absorbs enough energy, then the electron 'jumps' to a higher-energy orbit such as n=2. The atom is then in an unstable, excited state and quickly returns to the stable ground state when the electron jumps down from n=2 to n=1. It can do so only by emitting a quantum of energy that is equivalent to the difference in energy of the two levels, 10.2eV. The wavelength of the resulting spectral line can be calculated using the Planck-Einstein formula, E=hv, where v is the frequency of the emitted electromagnetic radiation.
An electron jumping from a range of higher energy levels to the same lower energy level produced the four spectral lines of the Balmer series. The size of the quanta emitted depended only on the initial and final energy levels involved. This was why Balmer's formula generated the correct wavelengths when n was set equal to 2 but m was 3, 4, 5 or 6 in turn. Bohr was able to derive the other spectral series predicted by Balmer by fixing the lowest energy level that the electron could jump to. For example, transitions ending with the electron jumping to n=3 produced the Paschen series in the infrared, while those that ended at n=1 generated the so-called Lyman series in the ultraviolet region of the spectrum.23
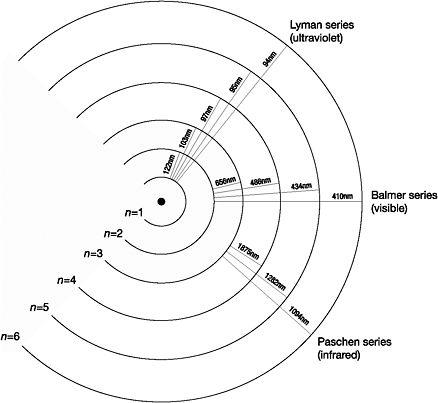
Figure 7: Energy levels, line spectra and quantum jumps (not drawn to scale)
There is, as Bohr discovered, a very strange feature associated with an electron's quantum leap. It is impossible to say where an electron actually is during a jump. The transition between orbits, energy levels, has to occur instantaneously. Otherwise as the electron travelled from one orbit to another it would radiate energy continuously. In Bohr's atom, an electron could not occupy the space between orbits. As if by magic, it disappeared while in one orbit and instantly reappeared in another.
'I'm fully convinced that the problem of spectral lines is intimately tied to the question of the nature of the quantum.' Remarkably, it was Planck, in February 1908, who wrote these words in a notebook.24 But in his ongoing struggle to minimise the impact of the quantum, and before the Rutherford atom, it was as far as Planck could go. Bohr embraced the idea that electromagnetic radiation was emitted and absorbed by atoms in quanta, but in 1913 he did not accept that electromagnetic radiation itself was quantised. Even six years later, in 1919, few believed in Einstein's quantum of light when Planck declared in his Nobel Prize lecture that Bohr's quantum atom was 'the long-sought key to the entrance-gate into the wonderland' of spectroscopy.25
![]()
On 6 March 1913, Bohr sent Rutherford the first of a trilogy of papers and asked him to send it on to the Philosophical Magazine. At the time, and for many years to come, every junior scientist like Bohr needed someone of Rutherford's seniority to 'communicate' a paper to a British journal to ensure swift publication. 'I am very anxious to know what you may think of it all', he wrote to Rutherford.26 He was particularly concerned about the reaction to his mixing of the quantum and classical physics. Bohr did not have to wait long for the answer: 'Your ideas as to the mode of origin of spectra in hydrogen are very ingenious and seem to work out well; but the mixture of Planck's ideas with the old mechanics make it very difficult to form a physical idea of what is the basis of it all.'27
Rutherford, as others would, was having trouble picturing how the electron in the hydrogen atom 'jumped' between energy levels. The difficulty lay in the fact that Bohr had violated a cardinal rule of classical physics. An electron moving in a circle is an oscillating system, with one complete orbit being an oscillation and the number of orbits per second being the frequency of oscillation. An oscillating system radiates energy at the frequency of its oscillation, but since two energy levels are involved in an electron making a 'quantum jump', there are two frequencies of oscillation. Rutherford was complaining that there was no link between these frequencies, between the 'old' mechanics and the frequency of the radiation emitted as the electron jumps between energy levels.
He also identified another more serious problem: 'There appears to me one grave difficulty in your hypothesis, which I have no doubt you fully realize, namely, how does an electron decide what frequency it is going to vibrate at when it passes from one stationary state to the other? It seems to me that you would have to assume that the electron knows beforehand where it is going to stop.'28 An electron in the n=3 energy level can jump down to either the n=2 or the n=1 levels. In order to make the jump, the electron appears to 'know' to which energy level it is heading so that it can emit radiation of the correct frequency. These were weakness of the quantum atom to which Bohr had no answer.
There was another, more minor criticism that concerned Bohr far more deeply. Rutherford thought the paper 'really ought to be cut down', since 'long papers have a way of frightening readers, who feel that they have not time to dip into them'.29 After offering to correct Bohr's English where necessary, Rutherford added a postscript: 'I suppose you have no objection to my using my judgement to cut out any matter I may consider unnecessary in your paper? Please reply.'30
When Bohr received the letter he was horrified. For a man who agonised over the choice of every word and went through endless drafts and revisions, the idea that someone else, even Rutherford, would make changes was appalling. Two weeks after posting the original paper, Bohr sent a longer revised manuscript containing alterations and additions. Rutherford agreed that the changes were 'excellent and appear quite reasonable', but he once again urged Bohr to cut the length. Even before he received this latest letter, he wrote to Rutherford telling him that he was coming to Manchester on holiday.31
When Bohr knocked on the front door, Rutherford was busy entertaining his friend Arthur Eve. He later recalled that Rutherford immediately took the 'slight-looking boy' into his study, leaving Mrs Rutherford to explain that the visitor was a young Dane and her husband thought 'very highly indeed of his work'.32 Through hour after hour of discussions over several long evenings during the days that followed, Bohr admitted that Rutherford 'showed an almost angelic patience' as he tried to defend every word in his paper.33
An exhausted Rutherford finally gave in and afterwards began regaling his friends and colleagues with tales of the encounter: 'I could see that he had weighed up every word in it, and it impressed me how determinedly he held on to every sentence, every expression, every quotation; everything had a definite reason, and although I first thought that many sentences could be omitted, it was clear, when he explained to me how closely knit the whole was, that it was impossible to change anything.'34 Ironically, Bohr admitted years later that Rutherford had been right 'in objecting to the rather complicated presentation'.35
Bohr's trilogy was published virtually unchanged in the Philosophical Magazine as 'On the Constitution of Atoms and Molecules'. The first, dated 5 April 1913, appeared in July. The second and third parts, published in September and November, were a presentation of ideas concerning the possible arrangements of electrons inside atoms that would preoccupy Bohr for the next decade as he used the quantum atom to explain the periodic table and the chemical properties of the elements.
![]()
Bohr had built his atom using a heady cocktail of classical and quantum physics. In the process he had violated tenets of accepted physics by proposing that: electrons inside atoms can occupy only certain orbits, the stationary states; electrons cannot radiate energy while in those orbits; an atom can be in only one of a series of discrete energy states, the lowest being the 'ground state'; electrons can 'somehow' jump from a stationary state of high energy to a stationary state of low energy and the difference in energy between the two is emitted in a quantum of energy. Yet his model correctly predicted various properties of the hydrogen atom such as its radius, and it provided a physical explanation for the production of spectral lines. The quantum atom, Rutherford said later, was 'a triumph of mind over matter' and until Bohr unveiled it, he believed that 'it would require centuries' to solve the mystery of the spectral lines.36
A true measure of Bohr's achievement was the initial reactions to the quantum atom. It was discussed publicly for the first time on 12 September 1913 at the 83rd annual meeting of the British Association for the Advancement of Science (BAAS), held that year in Birmingham. With Bohr in the audience, it received a muted and mixed reception. J.J. Thomson, Rutherford, Rayleigh and Jeans were all there, while the distinguished foreign contingent included Lorentz and Curie. 'Men over seventy should not be hasty in expressing opinions on new theories', was Rayleigh's diplomatic response when pressed for his opinion about Bohr's atom. In private, however, Rayleigh did not believe 'that Nature behaved in that way' and admitted that he had 'difficulty in accepting it as a picture of what actually takes place'.37 Thomson objected to Bohr's quantisation of the atom as totally unnecessary. James Jeans begged to differ. He pointed out in a report to the packed hall that the only justification that Bohr's model required was 'the very weighty one of success'.38
In Europe, the quantum atom was greeted with disbelief. 'This is all nonsense! Maxwell's equations are valid under all circumstances', said Max von Laue during one heated discussion. 'An electron in a circular orbit must emit radiation.'39 While Paul Ehrenfest confessed to Lorentz that Bohr's atom 'has driven me to despair'.40 'If this is the way to reach the goal,' he continued, 'I must give up doing physics.'41 In Göttingen, Bohr's brother Harald reported that there was great interest in his work, but that his assumptions were deemed too 'bold' and 'fantastic'.42
One early triumph for Bohr's theory clinched the support of some, including Einstein. Bohr predicted that a series of lines found in the spectrum of light from the sun that had been attributed to hydrogen actually belonged to ionised helium, helium with one of its two electrons removed. This interpretation of the so-called 'Pickering-Fowler lines' was at odds with that of its discoverers. Who was right? The issue was settled by one of Rutherford's team in Manchester after a detailed study of the spectral lines instigated at the behest of Bohr. Just in time for the BAAS meeting in Birmingham, it was found that the Dane had been correct in his assignment of the Pickering-Fowler lines to helium. Einstein heard the news during a conference in Vienna at the end of September from Bohr's friend Georg von Hevesy. 'The big eyes of Einstein,' reported Hevesy in a letter to Rutherford, 'looked still bigger and he told me: "Then it is one of the greatest discoveries".'43
By the time Part III of the trilogy was published in November 1913, another member of Rutherford's group, Henry Moseley, had confirmed the idea that the nuclear charge of an atom, its atomic number, was a unique whole number for a given element and the key parameter that decided its position within the periodical table. It was only after Bohr visited Manchester in July that year and spoke to Moseley about the atom that the young Englishman began shooting beams of electrons at different elements and examined the resulting X-ray spectra.
By then it was known that X-rays were a form of electromagnetic radiation with wavelengths thousands of times shorter than those of visible light, and that they were produced when electrons with sufficient energy struck a given metal. Bohr believed that X-rays were emitted because one of the innermost electrons was knocked out of an atom and an electron moved down from a higher energy level to fill the vacancy. The difference in the two energy levels was such that the quantum of energy emitted in the transition was an X-ray. Bohr realised that, using his atomic model, it was possible to determine the charge of the nucleus using the frequencies of the emitted X-rays. It was this intriguing fact that he had discussed with Moseley.
With a prodigious capacity for work matched only by his stamina, while others slept Moseley stayed in the laboratory working through the night. Within a couple of months he had measured the frequencies of X-rays emitted by every element between calcium and zinc. He discovered that as the elements he bombarded got heavier, there was a corresponding increase of frequencies of the emitted X-rays. Moseley predicted the existence of missing elements with atomic numbers 42, 43, 72 and 75 on the basis that each element produced a characteristic set of X-ray spectral lines and those adjacent to each other in the periodic table had very similar ones.44 All four were later discovered, but by then Moseley was dead. When the First World War began he enlisted in the Royal Engineers and served as a signals officer. He died, shot through the head, in Gallipoli on 10 August 1915. His tragic death at the age of 27 robbed him of a certain Nobel Prize. Rutherford personally gave him the highest possible accolade: he hailed Moseley as 'a born experimenter'.
Bohr's correct assignment of the 'Pickering-Fowler lines' and Moseley's ground-breaking work on nuclear charge were beginning to win support for the quantum atom. A more significant turning point in its acceptance came in April 1914, when the young German physicists James Franck and Gustav Hertz bombarded mercury atoms with electrons and found that the electrons lost 4.9eV of energy during these collisions. Franck and Hertz believed they had succeeded in measuring the amount of energy required to rip an electron from a mercury atom. Not having read his paper, due to the initial widespread scepticism that greeted it in Germany, it was left to Bohr to provide the correct interpretation of their data.
When the electrons fired at the mercury atoms had energies of less than 4.9eV, nothing happened. But when a bombarding electron with energy above 4.9eV scored a direct hit, it lost that amount of energy and the mercury atom emitted an ultraviolet light. Bohr pointed out that 4.9eV was the energy difference between the ground state of the mercury atom and its first excited state. It corresponded to an electron jumping between the first two energy levels in the mercury atom, and the energy difference between these levels was exactly as predicted by his atomic model. When the mercury atom returns to its ground state, as the electron jumps down to the first energy level, it emits a quantum of energy that produces an ultraviolet light of wavelength 253.7nm in the mercury line spectra. The Franck-Hertz results provided direct experimental evidence for Bohr's quantised atom and the existence of atomic energy levels. Despite initially having misinterpreted their data, Franck and Hertz were awarded the 1925 Nobel Prize in physics.
![]()
Just as Part I of the trilogy was published in July 1913, Bohr had finally been appointed to a lectureship at Copenhagen University. Before long he was unhappy, as his major responsibility was to teach elementary physics to medical students. At the beginning of 1914, with his reputation on the rise, Bohr set about trying to establish a new professorship in theoretical physics for himself. It would be difficult, as theoretical physics as a distinct discipline was still poorly recognised as such outside Germany. 'In my opinion Dr Bohr is one of the most promising and able of the young Mathematical Physicists in Europe today', wrote Rutherford in the testimonial to the Department of Religious and Educational Affairs in support of Bohr and his proposal.45 The immense interest that his work had attracted internationally ensured that Bohr received the backing of the faculty, but once again the university hierarchy chose to postpone any decision. It was then that a dejected Bohr received a letter from Rutherford offering an escape route.
'I daresay you know Darwin's tenure of readership has expired, and we are now advertising for a successor at £200', Rutherford wrote.46 'Preliminary inquiries show that not many men of promise are available. I should like to get a young fellow with some originality in him.' Having already told the Dane that his work showed 'great originality and merit', Rutherford wanted Bohr without explicitly saying so.47
In September 1914, having been granted a year's leave of absence, as any decision on the professorship he wanted was unlikely before then, Niels and Margrethe Bohr arrived in Manchester to a warm welcome at their safe arrival after a stormy voyage around Scotland. The First World War had begun and much had changed. The wave of patriotism that swept the country had virtually emptied the laboratories as those eligible to fight signed up. The hope that the war would be short and sharp receded by the day as the Germans smashed through Belgium and into France. Men who had only recently been colleagues were now fighting on opposing sides. Marsden was soon at the western front. Geiger and Hevesy had joined the armies of the Central Powers.
Rutherford was not in Manchester when Bohr arrived. He had left in June to attend the annual meeting of the British Association for the Advancement of Science, being held that year in Melbourne, Australia. Recently knighted, he visited his family in New Zealand before travelling on to America and Canada as planned. Once back in Manchester, Rutherford devoted much of his time to anti-submarine warfare. Since Denmark was neutral, Bohr was not allowed to take part in any war-related activities. He concentrated largely on teaching, and what research was possible was impeded by the lack of journals and the censorship of letters from and to Europe.
Originally planning to spend just a year in Manchester, Bohr was still there when in May 1916 he was formally appointed to the newly created post of professor of theoretical physics in Copenhagen. The growing recognition of his work had secured the post, but despite its successes there were problems that the quantum atom could not solve. The answers it gave for atoms with more than one electron failed to tally with experiments. It could not even account for helium with just two electrons. Worse, Bohr's atomic model predicted spectral lines that could not be found. In spite of the introduction of ad hoc 'selection rules' to explain why some lines were observed and others were not, all the central elements of Bohr's atom were accepted by the end of 1914: the existence of discrete energy levels, the quantisation of angular momentum of the orbiting electrons, and the origin of spectral lines. However, if there existed a single spectral line that could not be explained, even with the imposition of some new rule, then the quantum atom was in trouble.
In 1892, improved equipment appeared to show that the red alpha and blue gamma Balmer lines of the hydrogen spectrum were not single lines at all, but were each split in two. For more than twenty years, it remained an open question whether these lines were 'true doublets' or not. Bohr thought not. It was at the beginning of 1915 that he changed his mind as new experiments revealed that the red, blue and violet Balmer lines were all doublets. Using his atomic model, Bohr could not explain this 'fine structure', as the splitting of the lines was called. As he settled into his new role as a professor in Copenhagen, Bohr found a batch of papers waiting for him from a German who had solved the problem by modifying his atom.
Arnold Sommerfeld was a 48-year-old distinguished professor of theoretical physics at Munich University. Over the years, some of the most brilliant young physicists and students would work under his watchful eye as he turned Munich into a thriving centre of theoretical physics. Like Bohr, he loved skiing and would invite students and colleagues to his house in the Bavarian Alps to ski and talk physics. 'But let me assure you that if I were in Munich and had the time, I would sit in on your lectures in order to perfect my knowledge of mathematical physics', Einstein had written to Sommerfeld in 1908 while still at the Patent Office.48 It was some compliment coming from a man described as a 'lazy dog' by his maths professor in Zurich.
To simplify his model, Bohr had confined electrons to move only in circular orbits around the nucleus. Sommerfeld decided to lift this restriction, allowing electrons to move in elliptical orbits, like the planets in their journey around the sun. He knew that, mathematically speaking, circles were just a special class of ellipse, therefore circular electron orbits were only a subset of all possible quantised elliptical orbits. The quantum number n in the Bohr model specified a stationary state, a permitted circular electron orbit, and the corresponding energy level. The value of n also determined the radius of a given circular orbit. However, two numbers are required to encode the shape of an ellipse. Sommerfeld therefore introduced k, the 'orbital' quantum number, to quantise the shape of an elliptical orbit. Of all the possible shapes of an elliptical orbit, k determined those that were allowed for a given value of n.
In Sommerfeld's modified model, the principal quantum number n determined the values that k could have.49 If n=1, then k=1; when n=2, k=1 and 2; when n=3, k=1, 2 and 3. For a given n, k is equal to every whole number from 1 up to and including the value of n. When n=k, the orbit is always circular. However, if k is less than n, then the orbit is elliptical. For example, when n=1 and k=1, the orbit is circular with a radius r, called the Bohr radius. When n=2 and k=1, the orbit is elliptical; but n=2 and k=2 is a circular orbit with a radius 4r. Thus, when the hydrogen atom is in the n=2 quantum state, its single electron can be in either the k=1 or k=2 orbits. In the n=3 state, the electron can occupy any one of three orbits: n=3 and k=1, elliptical; n=3, k=2, elliptical; n=3 and k=3, circular. Whereas in Bohr's model n=3 was just one circular orbit, in Sommerfeld's modified quantum atom there were three permitted orbits. These extra stationary states could explain the splitting of the spectral lines of the Balmer series.
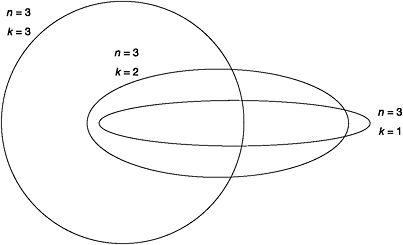
Figure 8: Electron orbits for n=3 and k=1, 2, 3 in the Bohr-Sommerfeld model of the hydrogen atom
To account for the splitting of the spectral lines, Sommerfeld turned to Einstein's theory of relativity. Like a comet in orbit about the sun, as an electron in an elliptical orbit heads towards the nucleus its speed increases. Unlike a comet, the speed of the electron is great enough for its mass to increase as predicted by relativity. This relativistic mass increase gives rise to a very small energy change. The n=2 states, the two orbits, k=1 and k=2, have different energies because k=1 is elliptical and k=2 circular. This minor energy difference leads to two energy levels that yield two spectral lines where only one was predicted by Bohr's model. However, the Bohr-Sommerfeld quantum atom was still unable to explain two other phenomena.
In 1897 the Dutch physicist Pieter Zeeman discovered that in a magnetic field, a single spectral line split into a number of separate lines or components. This was called the Zeeman effect, and once the magnetic field was switched off, the splitting disappeared. Then in 1913 the German physicist Johannes Stark found that a single spectral line splits up into several lines when atoms are placed in an electric field.50 Rutherford contacted Bohr as Stark published his findings: 'I think it is rather up to you at the present time to write something on the Zeeman and electric effects, if it is possible to reconcile them with your theory.'51
Rutherford was not the first to ask. Soon after the publication of Part I of his trilogy, Bohr had received a letter of congratulation from Sommerfeld. 'Will you also apply your atomic model to the Zeeman effect?' he asked. 'I want to tackle this.'52 Bohr was unable to explain it, but Sommerfeld did. His solution was ingenious. Earlier he had opted for elliptical orbits and thereby increased the number of possible quantised orbits that an electron could occupy when an atom was in a given energy state, such as n=2. Bohr and Sommerfeld had both pictured orbits, whether circular or elliptical, as lying in a plane. As he tried to account for the Zeeman effect, Sommerfeld realised that the orientation of an orbit was the vital missing component. In a magnetic field, an electron can select from more permitted orbits pointing in various directions with respect to the field. Sommerfeld introduced what he called the 'magnetic' quantum number m to quantise the orientation of those orbits. For a given principal quantum number n, m can only have values that range from -n to n.53 If n=2, then m has the values: -2, -1, 0, 1, 2.
'I do not believe ever to have read anything with more joy than your beautiful work', Bohr wrote to Sommerfeld in March 1916. The orientation of electron orbits, or 'space quantisation' as it became known, was experimentally confirmed five years later in 1921. It made available extra energy states, now labelled by the three quantum numbers n, k and m, which an electron could occupy in the presence of an external magnetic field, leading to the Zeeman effect.
Necessity being the mother of invention, Sommerfeld had been forced to introduce his two new quantum numbers k and m to explain facts revealed by experiments. Leaning heavily on the work of Sommerfeld, others explained the Stark effect as resulting from the changes in the spacing between energy levels due to the presence of an electric field. Although there were still weaknesses, such as the inability to reproduce the relative intensity of the spectral lines, the successes of the Bohr-Sommerfeld atom further enhanced Bohr's reputation and earned him an institute of his own in Copenhagen. He was on his way to becoming, as Sommerfeld called him later, 'the director of atomic physics' through his work and the inspiration he gave others.54
It was a compliment that would have pleased Bohr, who had always wanted to replicate the way in which Rutherford had run his laboratory, and the spirit he had succeeded in creating among all those who worked there. Bohr had learnt more than just physics from his mentor. He saw how Rutherford was able to galvanise a group of young physicists into producing their best. In 1917 Bohr set out to replicate what he had been fortunate enough to experience in Manchester. He approached the authorities in Copenhagen about establishing an institute for theoretical physics at the university. The institute was approved, as friends raised the money necessary for buildings and land. Construction began the following year, soon after the end of the war, at a site on the edge of a beautiful park not far from the city centre.
Work had only just begun when a letter arrived that unsettled Bohr. It was from Rutherford, who was offering him a permanent professorship in theoretical physics back in Manchester. 'I think the two of us could try and make physics boom', wrote Rutherford.55 It was tempting, but Bohr could not leave Denmark just as he was about to be given everything that he wanted. Maybe if he had gone, Rutherford would not have left Manchester in 1919 to take over from J.J. Thomson as the director of the Cavendish Laboratory at Cambridge.
Always known as the Bohr Institute, the Universitetets Institut for Teoretisk Fysik was formally opened on 3 March 1921.56 The Bohrs had already moved into the seven-room flat on the first floor with their growing family. Following the upheavals of war and the hardship of the years that followed in its wake, the institute was soon the creative haven Bohr hoped it would be. It quickly became a magnet for many of the world's brightest physicists, but the most talented of them all would always remain an outsider.