Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality - Manjit Kumar (2009)
Part I. THE QUANTUM
Chapter 2. THE PATENT SLAVE
Bern, Switzerland, Friday, 17 March 1905. It was nearly eight o'clock in the morning as the young man dressed in the unusual plaid suit hurried to work clutching an envelope. To a passer-by, Albert Einstein appeared to have forgotten that he was wearing a pair of wornout green slippers with embroidered flowers.1 At the same time six days a week, he left his wife and baby son, Hans Albert, behind in their small two-room apartment in the middle of Bern's picturesque Old Town quarter, and walked to the rather grand sandstone building ten minutes away. With its famous clock tower, the Zytloggeturm, and arcades lining both sides of the cobbled street, Kramgasse was one of the most beautiful streets in the Swiss capital. Lost in thought, Einstein hardly noticed his surroundings as he made his way to the administrative headquarters of the Federal Post and Telephone Service. Once inside he headed straight for the stairs and the third floor that housed the Federal Office of Intellectual Property, better known as the Swiss Patent Office. Here he and the dozen other technical experts, men in more sober dark suits, laboured at their desks for eight hours a day sorting out the barely viable from the fatally flawed.
Three days earlier, Einstein had celebrated his 26th birthday. He had been a 'patent slave', as he called it, for nearly three years.2 For him the job brought to an end 'the annoying business of starving'.3 The work itself he enjoyed for its variety, the 'many-sided thinking' it encouraged and the relaxed atmosphere of the office. It was an environment Einstein later referred to as his 'worldly monastery'. Although the post of technical expert, third class, was a humble one, it was well-paid and allowed him time enough to pursue his own research. Despite the watchful eye of his boss, the formidable Herr Haller, Einstein spent so much time between examining patents secretly doing his own calculations that his desk had become his 'office for theoretical physics'.4
'It was as if the ground had been pulled out from under one, with no firm foundation to be seen anywhere, upon which one could have built', was how Einstein recalled feeling after reading Planck's solution of the blackbody problem soon after it was published.5 What he sent in the envelope to the editor of Annalen der Physik, the world's leading physics journal, on 17 March 1905 was even more radical than Planck's original introduction of the quantum. Einstein knew that his proposal of a quantum theory of light was nothing short of heresy.
Two months later, in the middle of May, Einstein wrote to his friend Conrad Habicht promising to send four papers he hoped to see published before the year's end. The first was the quantum paper. The second was his PhD dissertation in which he set out a new way to determine the sizes of atoms. The third offered an explanation of Brownian motion, the erratic dance of tiny particles, like grains of pollen, suspended in liquid. 'The fourth paper,' Einstein admitted, 'is only a rough draft at this point and is an electrodynamics of moving bodies which employs a modification of the theory of space and time.'6 It is an extraordinary list. In the annals of science only one other scientist and one other year bears comparison with Einstein and his achievements in 1905: Isaac Newton in 1666, when the 23-year-old Englishman laid the foundations of calculus and the theory of gravity, and outlined his theory of light.
Einstein would become synonymous with the theory first sketched out in his fourth paper: relativity. Although it would change humanity's very understanding of the nature of space and time, it was the extension of Planck's quantum concept to light and radiation that he described as 'very revolutionary', not relativity.7 Einstein regarded relativity as simply a 'modification' of ideas already developed and established by Newton and others, whereas his concept of light-quanta was something totally new, entirely his own, and represented the greatest break with the physics of the past. Even for an amateur physicist it was sacrilegious.
For more than half a century it had been universally accepted that light was a wave phenomenon. In 'On a Heuristic Point of View Concerning the Production and Transformation of Light', Einstein put forward the idea that light was not made up of waves, but particle-like quanta. In his resolution of the blackbody problem Planck had reluctantly introduced the idea that energy was absorbed or emitted as quanta, in discrete lumps. However, he, like everyone else, believed that electromagnetic radiation itself was a continuous wave phenomenon, whatever the mechanism of how it exchanged energy when it interacted with matter. Einstein's revolutionary 'point of view' was that light, indeed all electromagnetic radiation, was not wavelike at all but chopped up into little bits, light-quanta. For the next twenty years, virtually no one but he believed in his quantum of light.
From the beginning Einstein knew it would be an uphill struggle. He signalled as much by including 'On a Heuristic Point of View' in the title of his paper. 'Heuristic', as defined by The Shorter Oxford English Dictionary, means 'serving to find out'. What he was offering physicists was a way to explain the unexplained when it came to light, not a fully worked-out theory derived from first principles. His paper was a signpost towards such a theory, but even that proved too much for those unprepared to travel to a destination in the opposite direction to the long-established wave theory of light.
Received by the Annalen der Physik between 18 March and 30 June, Einstein's four papers would transform physics in the years ahead. Remarkably, he also found the time and energy to write 21 book reviews for the journal during the course of the year. Almost as an afterthought, since he did not tell Habicht about it, he wrote a fifth paper. It contained the one equation that almost everyone would come to know, E=mc2. 'A storm broke loose in my mind', was how he described the surge of creativity that consumed him as he produced his breathtaking succession of papers during that glorious Bern spring and summer of 1905.8
Max Planck, the adviser on theoretical physics for the Annalen der Physik, was among the first to read 'On the Electrodynamics of Moving Bodies'. Planck was immediately won over by what he, and not Einstein, later called the theory of relativity. As for the quantum of light, though he profoundly disagreed with it, Planck allowed Einstein's paper to be published. As he did so he must have wondered about the identity of this physicist capable of the sublime and the ridiculous.
![]()
'The people of Ulm are mathematicians' was the unusual medieval motto of the city on the banks of the Danube in the south-western corner of Germany where Albert Einstein was born. It was an apt birthplace on 14 March 1879 for the man who would become the epitome of scientific genius. The back of his head was so large and distorted, his mother feared her newborn son was deformed. Later he took so long to speak that his parents worried he never would. Not long after the birth of his sister, and only sibling, Maja in November 1881, Einstein adopted the rather strange ritual of softly repeating every sentence he wanted to say until satisfied it was word-perfect before uttering it aloud. At seven, to the relief of his parents, Hermann and Pauline, he began to speak normally. By then the family had lived in Munich for six years, having moved so Hermann could open an electrical business in partnership with his younger brother Jakob.
In October 1885, with the last of the private Jewish schools in Munich closed for more than a decade, the six-year-old Einstein was sent to the nearest school. Not surprisingly in the heartland of German Catholicism, religious education formed an integral part of the curriculum, but the teachers, he recalled many years later, 'were liberal and did not make any denominational distinctions'.9 However liberal and accommodating his teachers may have been, the anti-Semitism that permeated German society was never buried too far beneath the surface, even in the schoolroom. Einstein never forgot the lesson in which his religious studies teacher told the class how the Jews had nailed Christ to the cross. 'Among the children,' Einstein recalled years later, 'anti-Semitism was alive especially in elementaryschool.'10 Not surprisingly, he had few, if any, school friends. 'I am truly a lone traveller and have never belonged to my country, my home, my friends, or even my immediate family, with my whole heart', he wrote in 1930. He called himself an Einspänner, a one-horse cart.
As a schoolboy he preferred solitary pursuits and enjoyed nothing more than constructing ever-taller houses of cards. He had the patience and tenacity, even as a ten-year-old, to build them as high as fourteen storeys. These traits, already such a fundamental part of his make-up, would allow him to pursue his own scientific ideas when others might have given up. 'God gave me the stubbornness of a mule,' he said later, 'and a fairly keen scent.'11 Though others disagreed, Einstein maintained he possessed no special talents, only a passionate curiosity. This quality that others had, however, coupled with his stubbornness, meant that he continued to seek the answer to almost childlike questions long after his peers were taught to stop even asking them. What would it be like to ride on a beam of light? It was trying to answer this question that set him on his decade-long path to the theory of relativity.
In 1888, aged nine, Einstein started at the Luitpold Gymnasium, and he later spoke bitterly of his days there. Whereas young Max Planck enjoyed and thrived under a strict, militaristic discipline focused on rote learning, Einstein did not. Despite resenting his teachers and their autocratic methods, he excelled academically even though the curriculum was orientated towards the humanities. He scored top marks in Latin and did well in Greek, even after being told by his teacher 'that nothing would ever become of him'.12
The stifling emphasis on mechanical learning at school, and during music lessons with tutors at home, was in stark contrast to the nurturing influence of a penniless Polish medical student. Max Talmud was 21, and Albert ten, when every Thursday he began dining with the Einsteins as they adopted their own version of an old Jewish tradition of inviting a poor religious scholar to lunch on the Sabbath. Talmud quickly recognised the inquisitive young boy as a kindred spirit. Before long the two would spend hours discussing the books that Talmud had given him to read or had recommended. They began with books on popular science that brought to an end what Einstein called his 'religious paradise of youth'.13
The years at a Catholic school and instruction at home by a relative on Judaism had left their mark. Einstein, to the surprise of his secular parents, had developed what he described as 'a deep religiosity'. He stopped eating pork, sang religious songs on the way to school, and accepted the biblical story of creation as an established fact. Then, as he devoured one book after another on science, came the realisation that much of the Bible could not be true. It unleashed what he called 'a fanatic freethinking coupled with the impression that youth is intentionally being deceived by the State through lies; it was a crushing impression'.14 It sowed the seeds of a lifelong suspicion of every kind of authority. He came to view the loss of his 'religious paradise' as the first attempt to free himself from 'the chains of the "merely personal", from an existence which is dominated by wishes, hopes and primitive feelings'.15
As he lost faith in the teachings of one sacred book, he began to experience the wonder of his sacred little geometry book. He was still at primary school when his Uncle Jakob introduced him to the rudiments of algebra and began posing problems for him to solve. By the time Talmud gave him a book on Euclid's geometry, Einstein was already well versed in mathematics not normally expected of a boy of twelve. Talmud was surprised at the speed with which Einstein worked through the book, proving the theorems and completing the exercises. Such was his zeal that during the summer vacation he mastered the mathematics to be taught the following year at school.
With a father and an uncle in the electrical industry, Einstein not only learnt about science through reading but was surrounded by the technology that its application could produce. It was his father who unwittingly introduced Einstein to the wonder and mystery of science. One day, as his son lay ill in bed with a fever, Hermann showed him a compass. The movement of the needle appeared so miraculous that the five-year-old trembled and grew cold at the thought that 'Something deeply hidden had to be behind things.'16
The Einstein brothers' electrical business initially prospered. They went from manufacturing electric devices to installing power and lighting networks. The future seemed bright as the Einsteins notched up one success after another, including the contract to provide the first electric lighting for Munich's famous Oktoberfest.17 But in the end the brothers were simply outgunned by the likes of Siemens and AEG. There were many small electrical firms that prospered and survived in the shadow of these giants, but Jakob was over-ambitious and Hermann too indecisive for their company to be one of them. Beaten but not bowed, the brothers decided that Italy, where electrification was just beginning, was the place to start afresh. So in June 1894 the Einsteins relocated to Milan. All except fifteen-year-old Albert who was left behind in the care of distant relatives to complete the three remaining years to graduation from the school he detested.
For the sake of his parents he pretended that everything was fine in Munich. However, he was increasingly troubled by the thought of compulsory military service. Under German law, if he remained in the country until his seventeenth birthday, Einstein would have no choice but to report for duty when the time came or be declared a deserter. Alone and depressed, he had to think of a way out, when suddenly the perfect opportunity arose.
Dr Degenhart, the teacher of Greek who thought Einstein would never amount to anything, was now also his form tutor. During a heated argument, Degenhart told Einstein he should leave the school. Requiring no further encouragement, he did just that after obtaining a medical certificate stating that he was suffering from exhaustion and required complete rest to recover. At the same time, Einstein secured a testimonial from his mathematics teacher that he had mastered the subject to a level required to graduate. It had taken him just six months to follow in the footsteps of his family and cross the Alps into Italy.
His parents tried to reason with him, but Einstein refused to go back to Munich. He had an alternative plan. He would stay in Milan and prepare for the entrance exams, the following October, of the Federal Polytechnikum in Zurich. Established in 1854, and renamed Eidgenossische Technische Hochschule (ETH) in 1911, the 'Poly' was not as prestigious as Germany's leading universities. However, it did not require graduation from a gymnasium as a precondition for entry. To be accepted, he explained to his parents, he just needed to pass its entrance exams.
They soon discovered the second part of their son's plan. He wanted to renounce his German nationality and thereby remove the possibility of ever being called up for military service by the Reich. Too young to do it himself, Einstein needed his father's consent. Hermann duly gave it and formally applied to the authorities for his son's release. It was January 1896 before they received official notification that Albert, at the cost of three marks, was no longer a German citizen. For the next five years he was legally stateless until he became a Swiss citizen. A renowned pacifist later in life, once he was granted his new nationality Einstein turned up for his Swiss army medical, on 13 March 1901, the day before his 22nd birthday. Fortunately, he was found unfit for military service because of sweaty flat feet and varicose veins.18 As a teenager back in Munich, it was not the thought of serving in the army that bothered him, but the prospect of donning a grey uniform on behalf of the militarism of the German Reich which he hated.
'The happy months of my sojourn in Italy are my most beautiful memories' was how Einstein, even after 50 years, recalled his new carefree existence.19 He helped his father and uncle with their electrical business and travelled here and there visiting friends and family. In the spring of 1895 the family moved to Pavia, just south of Milan, where the brothers opened a new factory that lasted little more than a year before it too closed. Although amid the upheaval he worked hard to prepare, Einstein failed the Poly entrance exams. Yet his mathematics and physics results were so impressive that the professor of physics invited him to attend his lectures. It was a tantalising offer, but for once Einstein took some sound advice. He had done so badly in languages, literature and history that the director of the Poly urged him to go back to school for another year and recommended one in Switzerland.
By the end of October Einstein was in Aarau, a town 30 miles west of Zurich. With its liberal ethos, the Aargau canton school provided a stimulating environment that enabled Einstein to thrive. The experience of boarding with the classics teacher and his family was to leave an indelible mark. Jost Winteler and his wife Pauline encouraged freethinking among their three daughters and four sons, and dinner each evening was always a lively and noisy affair. Before long the Wintelers became surrogate parents and he even referred to them as 'Papa Winteler' and 'Mama Winteler'. Whatever the old Einstein said later about being a lone traveller, the young Einstein needed people who cared about him and he for them. Soon it was September 1896 and exam time. Einstein passed easily and headed to Zurich and the Federal Polytechnikum.20
![]()
'A happy man is too satisfied with the present to dwell too much upon the future', Einstein had written at the start of a short essay called 'My Future Plans', during his two-hour French exam. But an inclination for abstract thinking and the lack of practical sense had led him to decide on a future as a teacher of mathematics and physics.21 So it was that Einstein found himself, in October 1896, the youngest of eleven new students entering the Poly's School for Specialised Teachers in the Mathematical and Science Subjects. He was one of the five seeking to qualify to teach maths and physics. The only woman among them was to be his future wife.
None of Albert's friends could understand why he was attracted to Mileva Maric. A Hungarian Serb, she was four years older and a bout of childhood tuberculosis had left her with a slight limp. During the first year they sat through the five compulsory maths courses and mechanics - the single physics course offered. Although he had devoured his little sacred book of geometry in Munich, Einstein was no longer interested in mathematics for its own sake. Hermann Minkowski, his maths professor at the Poly, recalled that Einstein had been a 'lazy dog'. It was not apathy but a failure to grasp, as Einstein later confessed, 'that the approach to a more profound knowledge of the basic principles of physics is tied up with the most intricate mathematical methods'.22 It was something he learnt the hard way in the years of research that followed. He regretted not having tried harder to get 'a sound mathematical education'.23
Fortunately, Marcel Grossmann, one of the other three besides Einstein and Mileva enrolled on the course, was a better mathematician and more studious than either of them. It would be to Grossmann that Einstein later turned for help as he struggled with the mathematics needed to formulate the general theory of relativity. The two quickly became friends as they talked 'about anything that might interest young people whose eyes were open'.24 Only a year older, Grossmann must have been an astute judge of character, for he was so impressed by his classmate that he took him home to meet his parents. 'This Einstein,' he told them, 'will one day be a very great man.'25
It was only by using Grossmann's excellent set of notes that he passed the intermediate exams in October 1898. In old age, Einstein could barely bring himself to contemplate what might have happened without Grossmann's help after he began skipping lectures. It had all been so different at the beginning of Heinrich Weber's physics course, when Einstein looked 'forward from one of his lectures to the next'.26 Weber, who was in his mid-fifties, could make physics come alive for his students, and Einstein conceded that he lectured on thermodynamics with 'great mastery'. But he became disenchanted because Weber did not teach Maxwell's theory of electromagnetism or any of the latest developments. Soon Einstein's independent streak and contemptuous manner began to alienate his professors. 'You're a smart boy', Weber told him. 'But you have one great fault: you do not let yourself be told anything.'27
When the final exams took place in July 1900 he came fourth out of five. Einstein felt coerced by the exams, and they had such a deterring effect upon him that afterwards he found 'the consideration of any scientific problems distasteful to me for an entire year'.28 Mileva was last, and the only one to fail. It was a bitter blow for the couple who were now affectionately calling each other 'Johonzel' (Johnny) and 'Doxerl' (Dollie). Another soon followed.
A future as a schoolteacher no longer appealed to Einstein. Four years in Zurich had given rise to a new ambition. He wanted to be a physicist. The chances of getting a full-time job at a university were slim even for the best students. The first step was an assistant's position with one of the professors at the Poly. None wanted him and Einstein began searching further afield. 'Soon I will have honoured all physicists from the North Sea to the Southern tip of Italy with my offer!' he wrote to Mileva in April 1901 while visiting his parents.29
One of those honoured was Wilhelm Ostwald, a chemist at the Leipzig University. Einstein wrote to him twice; both letters went unanswered. It must have been distressing for his father to watch his son's growing despair. Hermann, unknown to Albert then or later, took it upon himself to intervene. 'Please forgive a father who is so bold as to turn to you, esteemed Herr professor, in the interest of his son', he wrote to Ostwald.30 'All those in position to give a judgement in the matter, praise his talents; in any case, I can assure you that he is extraordinarily studious and diligent and clings with great love to his science.'31 The heartfelt plea went unanswered. Later Ostwald would be the first to nominate Einstein for the Nobel Prize.
Although anti-Semitism may have played a part, Einstein was convinced that it was Weber's poor references that were behind his failure to secure an assistantship. As he grew increasingly despondent, a letter from Grossmann held out the possibility of a decent, well-paying job. Grossmann senior had learnt of Einstein's desperate situation and wanted to help the young man whom his son held in such high regard. He strongly recommended Einstein for the next vacancy that arose to his friend Friedrich Haller, the director of the Swiss Patent Office in Bern. 'When I found your letter yesterday,' Einstein wrote to Marcel, 'I was deeply moved by your devotion and compassion which did not let you forget your old luckless friend.'32 After five years of being stateless, Einstein had recently acquired Swiss citizenship and was certain it would help when applying for the job.
Maybe his luck had changed at last. He was offered and accepted a temporary teaching job at the school in Winterthur, a small town less than twenty miles from Zurich. The five or six classes Einstein taught each morning left him free to pursue physics in the afternoon. 'I cannot tell you how happy I would feel in such a job', he wrote to Papa Winteler shortly before his time in Winterthur ended. 'I have completely given up my ambition to get a position at a university, since I see that even as it is, I have enough strength and desire left for scientific endeavour.'33 Soon that strength was put to the test when Mileva announced she was pregnant.
After failing the Poly exams a second time, Mileva returned to her parents in Hungary to await the arrival of the baby. Einstein took the news of the pregnancy in his stride. He had already entertained thoughts of becoming an insurance clerk and now vowed to find any job, no matter how humble, so that they could marry. When their daughter was born, Einstein was in Bern. He never saw Lieserl. What happened to her, whether she was given up for adoption or died in infancy, remains a mystery.
In December 1901, Friedrich Haller wrote to Einstein asking him to apply for a vacancy at the Patent Office that was about to be advertised.34 The long search for a permanent job seemed at an end as Einstein sent off his application before Christmas. 'All the time I rejoice in the fine prospects which are in store for us in the near future', he wrote to Mileva. 'Have I already told you how rich we will be in Bern?'35 Convinced that everything would be settled quickly, Einstein quit a year-long tutoring job at a private boarding school in Schaffhausen after only a few months.
![]()
Bern was home to some 60,000 people when Einstein arrived during the first week of February 1902. The medieval elegance of the Old Town quarter had changed little in the 500 years since it had been rebuilt following a fire that destroyed half the city. It was here that Einstein found a room on Gerechtigkeitgasse, not far from the city's famous bear pit.36 Costing 23 francs a month, it was anything but the 'large, beautiful room' he described to Mileva.37Not long after he unpacked his bags, Einstein went down to the local newspaper to place an advert offering his services as a private tutor of mathematics and physics. It appeared on Wednesday, 5 February and offered a free trial lesson. Within days it paid off. One of the students described his new tutor as 'about five foot ten, broad-shouldered, slightly stooped, a pale brown skin, a sensuous mouth, black moustache, nose slightly aquiline, radiant brown eyes, a pleasant voice, speaking French correctly but with a slight accent'.38
A young Romanian Jew, Maurice Solovine, also came across the advert as he read his newspaper walking down the street. A philosophy student at Bern University, Solovine was also interested in physics. Frustrated that a lack of mathematics was preventing him from gaining a deeper understanding of physics, he immediately made his way to the address given in the newspaper. When Solovine rang the bell, Einstein had found a kindred spirit. The student and tutor talked for two hours. They shared many of the same interests and after spending another half hour chatting in the street, they agreed to meet the following day. When they did, all thoughts of a structured lesson were forgotten amid a shared enthusiasm for exploring ideas. 'As a matter of fact, you don't have to be tutored in physics', Einstein told him on the third day.39 What Solovine liked about Einstein, as the two quickly became friends, was the care with which he outlined a topic or problem as lucidly as possible.
Before long, Solovine suggested that they read a particular book and then discuss it. Having done the same with Max Talmud in Munich as a schoolboy, Einstein thought it an excellent idea. Soon Conrad Habicht joined them. A friend from Einstein's aborted stint teaching at the boarding school in Schaffhausen, Habicht had moved to Bern to complete a mathematics thesis at the university. United by their enthusiasm for studying and clarifying the problems of physics and philosophy for their own satisfaction, the three men started calling themselves the 'Akademie Olympia'.
Even though Einstein came highly recommended by a friend, Haller had to make sure he was capable of doing the job. The ever-growing number of patent applications for all manner of electrical devices had made the hiring of a competent physicist to work alongside his engineers a necessity rather than a favour for a friend. Einstein impressed Haller sufficiently to be provisionally appointed a 'Technical Expert, Third Class' with a salary of 3,500 Swiss francs. At eight o'clock in the morning on 23 June 1902, Einstein reported for his first day as a 'respectable Federal ink pisser'.40
'As a physicist,' Haller told Einstein, 'you haven't a clue about blueprints.'41 Until he could read and assess technical drawings, there would be no permanent contract. Haller took it upon himself to teach Einstein what he needed to know, including the art of expressing himself clearly, concisely, and correctly. Although he had never taken kindly to being instructed as a schoolboy or student, he knew that he needed to learn all he could from Haller, 'a splendid character and a clever mind'.42 'One soon gets used to his rough manner', Einstein wrote. 'I hold him in very high regard.'43 As he proved his worth, Haller likewise came to respect his young protégé as a prized member of staff.
In October 1902, aged only 55, his father fell seriously ill. Einstein travelled to Italy to see him one last time. It was then, as he lay dying, that Hermann gave Albert his permission to marry Mileva - a prospect that he and Pauline had long opposed. With only Solovine and Habicht as witnesses, Einstein and Mileva married the following January in a civil ceremony at the Bern registrar's office. 'Marriage is,' Einstein said later, 'the unsuccessful attempt to make something lasting out of an incident.'44 But in 1903 he was just happy to have a wife that cooked, cleaned, and simply looked after him.45 Mileva had hoped for more.
The Patent Office took up 48 hours a week. From Monday to Saturday Einstein started at eight o'clock and worked until noon. Then it was lunch either at home or with a friend at a nearby café. He was back in the office from two until six. It left 'eight hours for fooling around' each day, and 'then there's also Sunday', he told Habicht.46 It was September 1904 before Einstein's 'provisional' position was made permanent with a pay rise of 400 francs. By the spring of 1906 Haller was so impressed with Einstein's ability to 'tackle technically very difficult patent applications' that he rated him as 'one of the valued experts at the office'.47 He was promoted to technical expert, second class.
'I will be grateful to Haller for as long as I live', Einstein had written to Mileva soon after moving to Bern in the expectation that a job at the Patent Office would sooner or later be his.48 And he was. But it was only much later that he recognised the extent of the influence that Haller and the Patent Office exerted on him: 'I might not have died, but I would have been intellectually stunted.'49 Haller demanded that every patent application be evaluated rigorously enough to withstand any legal challenge. 'When you pick up an application, think that anything the inventor says is wrong,' he advised Einstein, or else 'you will follow the inventor's way of thinking, and that will prejudice you. You have to remain critically vigilant.'50 Accidentally, Einstein had found a job that suited his temperament and honed his abilities. The critical vigilance he exercised in assessing an inventor's hopes and dreams, often on the basis of unreliable drawings and inadequate technical specifications, Einstein brought to bear on the physics that occupied him. The 'many-sided thinking' his job entailed he described as a 'veritable blessing'.51
'He had the gift of seeing a meaning behind inconspicuous, well-known facts which had escaped everyone else', recalled Einstein's friend and fellow theoretical physicist Max Born. 'It was this uncanny insight into the working of nature which distinguished him from all of us, not his mathematical skill.'52 Einstein knew that his mathematical intuition was not strong enough to differentiate what was really basic 'from the rest of the more or less dispensable erudition'.53 But when it came to physics, his nose was second to none. Einstein said he 'learned to scent out that which was able to lead to fundamentals and to turn aside from everything else, from the multitude of things which clutter up the mind and divert it from the essential'.54
His years at the Patent Office only heightened his sense of smell. As with the patents that inventors submitted, Einstein looked for subtle flaws and inconsistencies in the blueprints of the workings of nature put forward by physicists. When he found such a contradiction in a theory, Einstein probed it ceaselessly until it yielded a new insight resulting in its elimination or an alternative where none had existed before. His 'heuristic' principle that light behaved in certain instances as if it was made up of a stream of particles, light-quanta, was Einstein's solution to a contradiction at the very heart of physics.
![]()
Einstein had long accepted that everything was composed of atoms and that these discrete, discontinuous bits of matter possessed energy. The energy of a gas, for example, was the sum total of the energies of the individual atoms of which it was made up. The situation was entirely different when it came to light. According to Maxwell's theory of electromagnetism, or any wave theory, the energy of a light ray continuously spreads out over an ever-increasing volume like the waves radiating outwards from the point where a stone hits the surface of a pond. Einstein called it a 'profound formal difference' and it made him uneasy while stimulating his 'many-sided thinking'.55 He realised that the dichotomy between the discontinuity of matter and the continuity of electromagnetic waves would dissolve if light was also discontinuous, made up of quanta.56
The quantum of light emerged out of Einstein's review of Planck's derivation of the blackbody radiation law. He accepted that Planck's formula was correct, but his analysis revealed what Einstein had always suspected. Planck should have arrived at an entirely different formula. However, since he knew the equation he was looking for, Planck fashioned his derivation to get it. Einstein worked out exactly where Planck had gone astray. In his desperation to justify his equation that he knew to be in perfect agreement with experiments, Planck had failed to consistently apply the ideas and techniques he used or that were available to him. If he had done so, Einstein realised that Planck would have obtained an equation that did not agree with the data.
Lord Rayleigh had originally proposed this other formula in June 1900, but Planck had taken little, if any, notice of it. At the time he did not believe in the existence of atoms and therefore disapproved of Rayleigh's use of the equipartition theorem. Atoms are free to move in only three ways: up and down, back and forth, and side to side. Called a 'degree of freedom', each is an independent way in which an atom can receive and store energy. In addition to these three kinds of 'translational' motion, a molecule made up of two or more atoms has three types of rotational motion about the imaginary axes joining the atoms, giving a total of six degrees of freedom. According to the equipartition theorem, the energy of a gas should be distributed equally among its molecules and then divided equally among the different ways in which a molecule can move.
Rayleigh employed the equipartition theorem to divide up the energy of blackbody radiation among the different wavelengths of radiation present inside a cavity. It had been a flawless application of the physics of Newton, Maxwell and Boltzmann. Aside from a numerical error that was later corrected by James Jeans, there was a problem with what became known as the Rayleigh-Jeans law. It predicted a build-up of an infinite amount of energy in the ultraviolet region of the spectrum. It was a breakdown of classical physics that many years later, in 1911, was dubbed 'the ultraviolet catastrophe'. Thankfully it did not actually happen, for a universe bathed in a sea of ultraviolet radiation would have made human life impossible.
Einstein had derived the Rayleigh-Jeans law on his own and knew that the distribution of blackbody radiation that it forecast contradicted the experimental data and led to the absurdity of an infinite energy in the ultraviolet. Given that the Rayleigh-Jeans law tallied with the behaviour of blackbody radiation only at long wavelengths (very low frequencies), Einstein's point of departure was Wilhelm Wien's earlier blackbody radiation law. It was the only safe choice, even though Wien's law managed to replicate the behaviour of blackbody radiation only at short wavelengths (high frequencies) and failed at longer wavelengths (lower frequencies) of the infrared. Yet it had certain advantages that appealed to Einstein. He had no doubts about the soundness of its derivation, and it perfectly described at least a portion of the blackbody spectrum to which he would restrict his argument.
Einstein devised a simple but ingenious plan. A gas is just a collection of particles, and in thermodynamic equilibrium it is the properties of these particles that determine, for example, the pressure exerted by the gas at a given temperature. If there were similarities between the properties of blackbody radiation and the properties of a gas, then he could argue that electromagnetic radiation is itself particle-like. Einstein began his analysis with an imaginary blackbody that was empty. But unlike Planck, he filled it with gas particles and electrons. The atoms in the walls of the blackbody, however, contained other electrons. As the blackbody is heated, they oscillate with a broad range of frequencies resulting in the emission and absorption of radiation. Soon the interior of the blackbody is teeming with speeding gas particles and electrons, and the radiation emitted by the oscillating electrons. After a while, thermal equilibrium is reached when the cavity and everything inside it is at the same temperature T.
The first law of thermodynamics, that energy is conserved, can be translated to connect the entropy of a system to its energy, temperature and volume. It was now that Einstein used this law, Wien's law and Boltzmann's ideas to analyse how the entropy of blackbody radiation depended on the volume it occupied 'without establishing any model for the emission or propagation of radiation'.57 What he found was a formula that looked exactly like one describing how the entropy of a gas, made up of atoms, is dependent on the volume it occupies. Blackbody radiation behaved as if it was made up of individual particle-like bits of energy.
Einstein had discovered the quantum of light without having to use either Planck's blackbody radiation law or his method. In keeping Planck at arm's length, Einstein wrote the formula slightly differently but it meant and encoded the same information as E=hv, that energy is quantised, that it comes only in units of hv. Whereas Planck had only quantised the emission and absorption of electromagnetic radiation so that his imaginary oscillators would produce the correct spectral distribution of blackbody radiation, Einstein had quantised electromagnetic radiation, and therefore light, itself. The energy of a quantum of yellow light was just Planck's constant multiplied by the frequency of yellow light.
By showing that electromagnetic radiation sometimes behaves like the particles of a gas, Einstein knew that he had smuggled his light-quanta in through the back door, by analogy. To convince others of the 'heuristic' value of his new 'point of view' concerning the nature of light, he used it to explain a little-understood phenomenon.58
The German physicist Heinrich Hertz first observed the photoelectric effect in 1887 while in the middle of performing a series of experiments that demonstrated the existence of electromagnetic waves. By chance he noticed that the spark between two metal spheres became brighter when one of them was illuminated by ultraviolet light. After months of investigating the 'completely new and very puzzling phenomenon' he could offer no explanation, but believed, incorrectly, that it was confined to the use of ultraviolet light.59
'Naturally, it would be nice if it were less puzzling,' Hertz admitted, 'however, there is some hope that when this puzzle is solved, more new facts will be clarified than if it were easy to solve.'60 It was a prophetic statement, but one that he never lived to see fulfilled. He died tragically young at the age of 36 in 1894.
It was Hertz's former assistant, Philipp Lenard, who in 1902 deepened the mystery surrounding the photoelectric effect when he discovered that it also occurred in a vacuum when he placed two metal plates in a glass tube and removed the air. Connecting the wires from each plate to a battery, Lenard found that a current flowed when one of the plates was irradiated with ultraviolet light. The photoelectric effect was explained as the emission of electrons from the illuminated metal surface. Shining ultraviolet light onto the plate gave some electrons enough energy to escape from the metal and cross the gap to the other plate, thereby completing the circuit to produce a 'photoelectric current'. However, Lenard also found facts that contradicted established physics. Enter Einstein and his quantum of light.
It was expected that increasing the intensity of a light beam, by making it brighter, would yield the same number of electrons from the metal surface, but with each having more energy. Lenard, however, found the exact opposite: a greater number of electrons were emitted with no change in their individual energy. Einstein's quantum solution was simple and elegant: if light is made up of quanta, then increasing the intensity of the beam means that it is now made up of a greater number of quanta. When a more intense beam strikes the metal plate, the increase in the number of light-quanta leads to a corresponding increase in the number of electrons being emitted.
Lenard's second curious discovery was that the energy of the emitted electrons was not governed by the intensity of the light beam, but by its frequency. Einstein had a ready answer. Since the energy of a light-quantum is proportional to the frequency of the light, a quantum of red light (low frequency) has less energy than one of blue light (high frequency). Changing the colour (frequency) of light does not alter the number of quanta in beams of the same intensity. So, no matter what the colour of light, the same number of electrons will be emitted since the same numbers of quanta strike the metal plate. However, since different frequencies of light are made up of quanta of different energies, the electrons that are emitted will have more or less energy depending on the light used. Ultraviolet light will yield electrons with a greater maximum kinetic energy than those emitted by quanta of red light.
There was another intriguing feature. For any particular metal there was a minimum or 'threshold frequency' below which no electrons were emitted at all, no matter how long or intensively the metal was illuminated. However, once this threshold was crossed, electrons were emitted no matter how dim the beam of light. Einstein's quantum of light supplied the answer once again as he introduced a new concept, the work function.
Einstein envisaged the photoelectric effect as the result of an electron acquiring enough energy from a quantum of light to overcome the forces holding it within the metal surface and to escape. The work function, as Einstein labelled it, was the minimum energy an electron needed to escape from the surface, and it varied from metal to metal. If the frequency of light is too low, then the light-quanta will not possess enough energy to allow an electron to break the bonds that keep it bound within the metal.
Einstein encoded all this in a simple equation: the maximum kinetic energy of an electron emitted from a metal surface was equal to the energy of the light-quanta it absorbed minus the work function. Using this equation, Einstein predicted that a graph of the maximum kinetic energy of the electrons versus the frequency of light used would be a straight line, beginning at the threshold frequency of the metal. The gradient of the line, irrespective of the metal used, would always be exactly equal to Planck's constant, h.
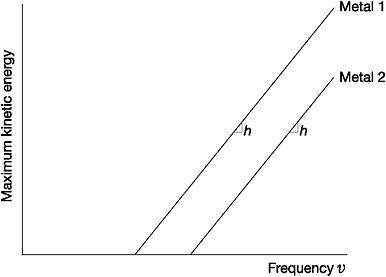
Figure 3: The photoelectric effect - maximum kinetic energy of emitted electrons versus the frequency of light striking the metal surface
'I spent ten years of my life testing that 1905 equation of Einstein's and contrary to all my expectations,' complained the American experimental physicist Robert Millikan, 'I was compelled to assert its unambiguous verification in spite of its unreasonableness, since it seemed to violate everything we knew about the interference of light.'61 Although Millikan won the 1923 Nobel Prize partly in recognition of this work, even in the face of his own data he balked at the underlying quantum hypothesis: 'the physical theory upon which the equation is based is totally untenable.'62 From the very beginning, physicists at large had greeted Einstein's light-quanta with similar disbelief and cynicism. A handful wondered if light-quanta existed at all or whether they were simply a useful fictional contrivance of practical value in calculations. At best some thought that light, and therefore all electromagnetic radiation, did not consist of quanta, but only behaved as such when exchanging energy with matter.63 Foremost among them was Planck.
When in 1913 he and three others nominated Einstein for membership of the Prussian Academy of Sciences, they concluded their testimonial by trying to excuse his light-quanta proposal: 'In sum, it can be said that among the important problems, which are so abundant in modern physics, there is hardly one in which Einstein did not take a position in a remarkable manner. That he might sometimes have overshot the target in his speculations, as for example in his light-quantum hypothesis, should not be counted against him too much. Because without taking a risk from time to time it is impossible, even in the most exact natural science, to introduce real innovations.'64
Two years later, Millikan's painstaking experiments made it difficult to ignore the validity of Einstein's photoelectric equation. By 1922 it was becoming almost impossible, as Einstein was belatedly awarded the 1921 Nobel Prize for physics explicitly for his photoelectric effect law, described by his formula, and not for his underlying explanation using light-quanta. No longer the unknown patent clerk in Bern, he was by then world-famous for his theories of relativity and widely acknowledged as the greatest scientist since Newton. Yet his quantum theory of light was just too radical for physicists to accept.
![]()
The stubborn opposition to Einstein's idea of light-quanta rested on the overwhelming evidence in support of a wave theory of light. However, whether light was a particle or a wave had been hotly disputed before. During the eighteenth century and in the early years of the nineteenth, it was Isaac Newton's particle theory that had triumphed. 'My Design in this Book is not to explain the Properties of Light by Hypotheses,' Newton wrote at the beginning of Opticks, published in 1704, 'but to propose and prove them by Reason and Experiments.'65 Those first experiments were conducted in 1666, when he split light into the colours of the rainbow with a prism and wove them back together into white light using a second prism. Newton believed that rays of light were composed of particles or, as he called them, 'corpuscles', the 'very small Bodies emitted from shining Substances'.66 With the particles of light travelling in straight lines, such a theory would, according to Newton, explain the everyday fact that while a person can be heard talking around a corner, they cannot be seen, since light cannot not bend around corners.
Newton was able to give a detailed mathematical account for a host of optical observations, including reflection and refraction - the bending of light as it passes from a less to a more dense medium. However, there were other properties of light that Newton could not explain. For example, when a beam of light hit a glass surface, part of it passed through and the rest was reflected. The question Newton had to address was why some particles of light were reflected and others not? To answer it, he was forced to adapt his theory. Light particles caused wavelike disturbances in the ether. These 'Fits of easy Reflexion and easy Transmission', as he called them, were the mechanism by which some of the beam of light was transmitted through the glass and the remainder reflected.67 He linked the 'bigness' of these disturbances to colour. The biggest disturbances, those having the longest wavelength, in the terminology that came later, were responsible for producing red. The smallest, those having the shortest wavelength, produced violet.
The Dutch physicist Christiaan Huygens argued that there was no Newtonian particle of light. Thirteen years older than Newton, by 1678 Huygens had developed a wave theory of light that explained reflection and refraction. However, his book on the subject, Traité de la Lumière, was not published until 1690. Huygens believed that light was a wave travelling through the ether. It was akin to the ripples that fanned out across the still surface of a pond from a dropped stone. If light was really made up of particles, Huygens asked, then where was the evidence of collisions that should occur when two beams of light crossed each other? There was none, argued Huygens. Sound waves do not collide; ergo light must also be wavelike.
Although the theories of Newton and Huygens were able to explain reflection and refraction, each predicted different outcomes when it came to certain other optical phenomena. None could be tested with any degree of precision for decades. However, there was one prediction that could be observed. A beam of light made up of Newton's particles travelling in straight lines should cast sharp shadows when striking objects, whereas Huygens' waves, like water waves bending around an object they encounter, should result in shadows whose outline is slightly blurred. The Italian Jesuit and mathematician Father Francesco Grimaldi christened this bending of light around the edge of an object, or around the edges of an extremely narrow slit, diffraction. In a book published in 1665, two years after his death, he described how an opaque object placed in a narrow shaft of sunlight allowed to enter an otherwise darkened room through a very small hole in a window shutter, cast a shadow larger than expected if light consisted of particles travelling in straight lines. He also found that around the shadow were fringes of coloured light and fuzziness where there should have been a sharp, well-defined separation between light and dark.
Newton was well aware of Grimaldi's discovery and later conducted his own experiments to investigate diffraction, which seemed more readily explicable in terms of Huygens' wave theory. However, Newton argued that diffraction was the result of forces exerted on light particles and indicative of the nature of light itself. Given his pre-eminence, Newton's particle theory of light, though in truth a strange hybrid of particle and wave, was accepted as the orthodoxy. It helped that Newton outlived Huygens, who died in 1695, by 32 years. 'Nature and Nature's Laws lay hid in Night; / God said, Let Newton be! And all was Light.' Alexander Pope's famous epitaph bears witness to the awe in which Newton was held in his own day. In the years after his death in 1727, Newton's authority was undiminished and his view on the nature of light barely questioned. At the dawn of the nineteenth century the English polymath, Thomas Young, did challenge it, and in time his work led to a revival of the wave theory of light.
Born in 1773, Young was the eldest of ten children. He was reading fluently by the age of two and had read the entire Bible twice by six. A master of more than a dozen languages, Young went on make important contributions towards the deciphering of Egyptian hieroglyphics. A trained physician, he could indulge his myriad intellectual pursuits after a bequest from an uncle left him financially secure. His interest in the nature of light led Young to examine the similarities and differences between light and sound, and ultimately to 'one or two difficulties in the Newtonian system'.68 Convinced that light was a wave, he devised an experiment that was to prove the beginning of the end for Newton's particle theory.
Young shone monochromatic light onto a screen with a single slit. From this slit a beam of light spread out to strike a second screen with two very narrow and parallel slits close together. Like a car's headlights, these two slits acted as new sources of light, or as Young wrote, 'as centres of divergence, from whence the light diffracted in every direction'.69 What Young found on another screen placed some distance behind the two slits was a central bright band surrounded on each side by a pattern of alternating dark and bright bands.
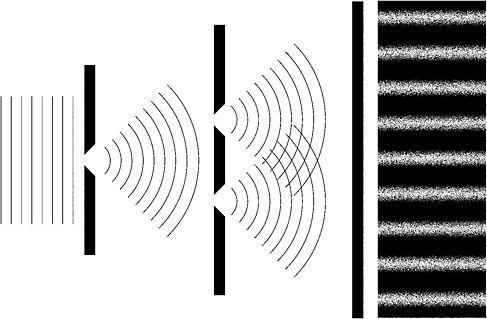
Figure 4: Young's two-slits experiment. At far right, the resulting interference pattern on the screen is shown
To explain the appearance of these bright and dark 'fringes', Young used an analogy. Two stones are dropped simultaneously and close together into a still lake. Each stone produces waves that spread out across the lake. As they do so, the ripples originating from one stone encounter those from the other. At each point where two wave troughs or two wave crests meet, they coalesce to produce a new single trough or crest. This was constructive interference. But where a trough meets a crest or vice versa, they cancel each other out, leaving the water undisturbed at that point - destructive interference.
In Young's experiment, light waves originating from the two slits similarly interfere with each other before striking the screen. The bright fringes indicate constructive interference while the dark fringes are a product of destructive interference. Young recognised that only if light is a wave phenomenon could these results be explained. Newton's particles would simply produce two bright images of the slits with nothing but darkness in between. An interference pattern of bright and dark fringes was simply impossible.
When he first put forward the idea of interference and reported his early results in 1801, Young was viciously attacked in print for challenging Newton. He tried to defend himself by writing a pamphlet in which he let everyone know his feelings about Newton: 'But, much as I venerate the name of Newton, I am not therefore obliged to believe that he was infallible. I see, not with exultation, but with regret, that he was liable to err, and that his authority has, perhaps, sometimes even retarded the progress of science.'70 Only a single copy was sold.
It was a French civil engineer who followed Young in stepping out of Newton's shadow. Augustin Fresnel, fifteen years his junior, independently rediscovered interference and much else of what Young, unknown to him, had already done. However, compared to the Englishman, Fresnel's elegantly designed experiments were more extensive, with the presentation of results and accompanying mathematical analysis so impeccably thorough that the wave theory started to gain distinguished converts by the 1820s. Fresnel convinced them that the wave theory could better explain an array of optical phenomena than Newton's particle theory. He also answered the long-standing objection to the wave theory: light cannot travel around corners. It does, he said. However, since light waves are millions of times smaller than sound waves, the bending of a beam of light from a straight path is very, very small and therefore extremely difficult to detect. A wave bends only around an obstacle not much longer than itself. Sound waves are very long and can easily move around most barriers they encounter.
One way to get opponents and sceptics to finally decide between the two rival theories was to find observations for which they predicted different results. Experiments conducted in France in 1850 revealed that the speed of light was slower in a dense medium such as glass or water than in the air. This was exactly what the wave of light predicted, while Newton's corpuscles failed to travel as fast as expected. But the question remained: if light was a wave, what were its properties? Enter James Clerk Maxwell and his theory of electromagnetism.
Born in 1831 in Edinburgh, Maxwell, the son of a Scottish landowner, was destined to become the greatest theoretical physicist of the nineteenth century. At the age of fifteen, he wrote his first published paper on a geometrical method for tracing ovals. In 1855 he won Cambridge University's Adams Prize for showing that Saturn's rings could not be solid, but had to be made of small, broken bits of matter. In 1860 he instigated the final phase of the development of the kinetic theory of gases, the properties of gases explained by maintaining that they consisted of particles in motion. But his greatest achievement was the theory of electromagnetism.
In 1819 the Danish physicist Hans Christian Oersted discovered that an electric current flowing through a wire deflected a compass needle. A year later the Frenchman François Arago found that a wire carrying an electric current acted as a magnet and could attract iron filings. Soon his compatriot André Marie Ampère demonstrated that two parallel wires were attracted towards one another if each had a current flowing through it in the same direction. However, they repelled each other if the currents flowed in the opposite directions. Intrigued by the fact that a flow of electricity could create magnetism, the great British experimentalist Michael Faraday decided to see if he could generate electricity using magnetism. He pushed a bar magnet in and out of a helix coil of wire and found an electric current being generated. The current ceased whenever the magnet was motionless within the coil.
Just as ice, water and steam are different manifestations of H2O, Maxwell showed in 1864 that electricity and magnetism were likewise different manifestations of the same underlying phenomenon - electromagnetism. He managed to encapsulate the disparate behaviour of electricity and magnetism into a set of four elegant mathematical equations. On seeing them, Ludwig Boltzmann immediately recognised the magnitude of Maxwell's achievement and could only quote Goethe in admiration: 'Was it a God that wrote these signs?'71 Using these equations, Maxwell was able to make the startling prediction that electromagnetic waves travelled at the speed of light through the ether. If he was right, then light was a form of electromagnetic radiation. But did electromagnetic waves actually exist? If so, did they really travel at the speed of light? Maxwell did not live long enough to see his prediction confirmed by experiment. Aged just 48, he died from cancer in November 1879, the year Einstein was born. Less than a decade later, in 1887, Heinrich Hertz provided the experimental corroboration that ensured Maxwell's unification of electricity, magnetism and light was the crowning achievement of nineteenth-century physics.
Hertz proclaimed in his paper outlining his investigations: 'The experiments described appear to me, at any rate, eminently adapted to remove any doubt as to the identity of light, radiant heat, and electromagnetic wave motion. I believe that from now on we shall have greater confidence in making use of the advantages, which this identity enables us to derive both in the study of optics and electricity.'72 Ironically, it was during these very experiments that Hertz discovered the photoelectric effect that provided Einstein with evidence for a case of mistaken identity. His light-quanta challenged the wave theory of light that Hertz and everyone else thought was well and truly established. Light as a form of electromagnetic radiation had proved so successful that for physicists to even contemplate discarding it in favour of Einstein's light-quanta was unthinkable. Many found light-quanta absurd. After all, the energy of a particular quantum of light was determined by the frequency of that light, but surely frequency was something associated with waves, not particle-like bits of energy travelling through space.
Einstein readily accepted that the wave theory of light had 'proved itself superbly' in explaining diffraction, interference, reflection and refraction, and that it would 'probably never be replaced by another theory'.73 However, this success, he pointed out, rested on the vital fact that all these optical phenomena involved the behaviour of light over a period of time, and any particle-like properties would not be manifest. The situation was starkly different when it came to the virtually 'instantaneous' emission and absorption of light. This was the reason, Einstein suggested, why the wave theory faced 'especially great difficulties' explaining the photoelectric effect.74
A future Nobel laureate, but in 1906 a privatdozent at Berlin University, Max Laue wrote to Einstein that he was willing to accept that quanta may be involved during the emission and absorption of light. However, that was all. Light itself was not made up of quanta, warned Laue, but it is 'when it is exchanging energy with matter that it behaves as if it consisted of them'.75 Few even conceded that much. Part of the problem lay with Einstein himself. In his original paper he did say that light 'behaves' as though it consisted of quanta. This was hardly a categorical endorsement of the quantum of light. This was because Einstein wanted something more than just a 'heuristic point of view': he craved a fully-fledged theory.
The photoelectric effect had proved to be a battlefield for the clash between the supposed continuity of light waves and the discontinuity of matter, atoms. But in 1905 there were still those who doubted the reality of atoms. On 11 May, less than two months after he finished his quantum paper, the Annalen der Physik received Einstein's second paper of the year. It was his explanation of Brownian motion and it became a key piece of evidence in support of the existence of atoms.76
When in 1827 the Scottish botanist Robert Brown peered through a microscope at some pollen grains suspended in water, he saw that they were in a constant state of haphazard motion as if buffeted by some unseen force. It had already been noted by others that this erratic wiggling increased as the temperature of the water rose, and it was assumed that some sort of biological explanation lay behind the phenomenon. However, Brown discovered that when he used pollen grains that were up to twenty years old they moved in exactly the same way. Intrigued, he produced fine powders of all manner of inorganic substances, from glass to a piece of the Sphinx, and suspended each of them in water. He found the same zigzagging motion in each case and realised that it could not be animated by some vital force. Brown published his research in pamphlet entitled: A Brief Account of Microscopical Observations Made in the Months of June, July, and August 1827, on the Particles Contained in the Pollen of Plants; and on the General Existence of Active Molecules in Organic and Inorganic Bodies. Others offered plausible explanations of 'Brownian motion', but all were sooner or later found wanting. By the end of the nineteenth century, those who believed in the existence of atoms and molecules accepted that Brownian motion was the result of collisions with water molecules.
What Einstein recognised was that the Brownian motion of a pollen grain was not caused by a single collision with a water molecule, but was the product of a large number of such collisions. At each moment, the collective effect of these collisions was the random zigzagging of the pollen grain or suspended particle. Einstein suspected that the key to understanding this unpredictable motion lay in deviations, statistical fluctuations, from the expected 'average' behaviour of water molecules. Given their relative sizes, on average, many water molecules would strike an individual pollen grain simultaneously from different directions. Even on this scale, each collision would result in an infinitesimal push in one direction, but the overall effect of all of them would leave the pollen unmoved as they cancelled each other out. Einstein realised that Brownian motion was due to water molecules regularly deviating from their 'normal' behaviour as some of them got bunched up and struck the pollen together, sending it in particular direction.
Using this insight, Einstein succeeded in calculating the average horizontal distance a particle would travel as it zigzagged along in a given time. He predicted that in water at 17°C, suspended particles with a diameter of one-thousandth of a millimetre would move on average just six-thousandths of a millimetre in one minute. Einstein had come up with a formula that offered the possibility of working out the size of atoms armed only with a thermometer, microscope and stopwatch. Three years later, in 1908, Einstein's predictions were confirmed in a delicate series of experiments conducted at the Sorbonne by Jean Perrin, for which he received the Nobel Prize in 1926.
![]()
With Planck championing the theory of relativity, and the analysis of Brownian motion recognised as a decisive breakthrough in favour of the atom, Einstein's reputation grew despite the rejection of his quantum theory of light. He received letters often addressed to him at Bern University, as few knew he was a patent clerk. 'I must tell you quite frankly that I was surprised to read that you must sit in an office for 8 hours a day,' wrote Jakob Laub from Würzburg. 'History is full of bad jokes.'77 It was March 1908 and Einstein agreed. After almost six years he no longer wanted to be a patent slave.
He applied for a job as a mathematics teacher at a school in Zurich, stating that he would be ready and willing to teach physics as well. With his application he enclosed a copy of his thesis that had earned him, at the third attempt, a doctorate from Zurich University in 1905 and laid the groundwork for the paper on Brownian motion. Hoping it would bolster his chances, he also sent all of his published papers. Despite his impressive scientific achievements, of the 21 applicants, Einstein did not even make the short list of three.
It was at the behest of Alfred Kleiner, the professor of experimental physics at Zurich University, that Einstein tried for a third time to become a privatdozent, an unpaid lecturer, at the University of Bern. The first application was rejected because at the time he did not have a PhD. In June 1907, he failed a second time because he did not submit a habilitationsschrift - a piece of unpublished research. Kleiner wanted Einstein to fill a soon-to-be-created extraordinary professorship in theoretical physics, and being a privatdozent was a necessary stepping-stone to such an appointment. So he produced a habilitationsschrift as demanded and was duly appointed a privatdozent in the spring of 1908.
Only three students attended his first lecture course on the theory of heat. All three were friends. They had to be, since Einstein had been allocated Tuesdays and Saturdays between seven and eight in the morning. University students had the choice of whether or not to attend courses offered by a privatdozent and none were willing to get up that early. As a lecturer, then and later, Einstein was often under-prepared and made frequent mistakes. And when he did, he simply turned to the students and asked: 'Who can tell me where I went wrong?' or 'Where have I made a mistake?' If a student pointed out an error in his mathematics, Einstein would say, 'I have often told you, my mathematics have never been up to much.'78
The ability to teach was a vital consideration for the job earmarked for Einstein. To ensure that he was up to the task, Kleiner organised to attend one of his lectures. Annoyed at 'having-to-be-investigated', he performed poorly.79However, Kleiner gave him a second chance to impress and he did. 'I was lucky', Einstein wrote to his friend Jakob Laub. 'Contrary to my habit, I lectured well on that occasion - and so it came to pass.'80 It was May 1909 and Einstein could finally boast that he was 'an official member of the guild of whores' as he accepted the Zurich post.81 Before moving to Switzerland with Mileva and five-year-old Hans Albert, Einstein travelled to Salzburg in September to give the keynote lecture to the cream of German physics at a conference of the Gesellschaft Deutscher Naturforscher und Ärtze. He went well prepared.
It was a singular honour to be asked to deliver such a lecture. It was one usually reserved for a distinguished elder statesman of physics, not someone who had just turned 30 and was about take up his first extraordinary professorship. So all eyes were on Einstein, but he seemed oblivious as he paced the podium and delivered what would turn out to be a celebrated lecture: 'On the Development of Our Views Concerning the Nature and Constitution of Radiation'. He told the audience that 'the next stage in the development of theoretical physics will bring us a theory of light that may be conceived of as a sort of fusion of the wave and of the emission theory of light'.82 It was not a hunch, but based on the result of an inspired thought experiment involving a mirror suspended inside a blackbody. He managed to derive an equation for the fluctuations of the energy and momentum of radiation that contained two very distinct parts. One corresponded to the wave theory of light, while the other had all the hallmarks of the radiation being composed of quanta. Both parts appeared to be indispensable, as did the two theories of light. It was the first prediction of what would later be called wave-particle duality - that light was both a particle and a wave.
Planck, who was chairing, was the first to speak after Einstein sat down. He thanked him for the lecture and then told everyone he disagreed. He reiterated his firmly held belief that quanta were necessary only in the exchange between matter and radiation. To believe as Einstein did that light was actually made up of quanta, Planck said, was 'not yet necessary'. Only Johannes Stark stood up to support Einstein. Sadly, he, like Lenard, would later become a Nazi and the two of them would attack Einstein and his work as 'Jewish Physics'.
![]()
Einstein left the Patent Office to devote more of his time to research. He was in for a rude awakening when he arrived in Zurich. The time he needed to prepare for the seven hours of lectures that he gave each week left him complaining that his 'actual free time is less than in Bern'.83 The students were struck by the shabby appearance of their new professor, but Einstein quickly gained their respect and affection by his informal style as he encouraged them to interrupt if anything was unclear. Outside formal lectures, at least once a week he took his students along to the Café Terasse to chat and gossip until closing time. Before long he got used to his workload and turned his attention to using the quantum to solve a longstanding problem.
In 1819 two French scientists, Pierre Dulong and Alexis Petit, measured the specific heat capacity, the amount of energy needed to raise the temperature of a kilogram of a substance by one degree, for various metals from copper to gold. For the next 50 years no one who believed in atoms doubted their conclusion that 'the atoms of all simple bodies have exactly the same heat capacity'.84 It therefore came as a great surprise when, in the 1870s, exceptions were discovered.
Imagining that the atoms of a substance oscillated when heated, Einstein adapted Planck's approach as he tackled the specific heat anomalies. Atoms could not oscillate with just any frequency, but were 'quantised' - able to oscillate only with those frequencies that were multiples of a certain 'fundamental' frequency. Einstein came up with a new theory of how solids absorb heat. Atoms are permitted to absorb energy only in discrete amounts, quanta. However, as the temperature drops, the amount of energy the substance has decreases, until there is not enough available to provide each atom with the correct-sized quantum of energy. This results in less energy being taken up by the solid and leads to a decrease in specific heat.
For three years there was hardly a murmur of interest in what Einstein had done, despite the fact that he had shown how the quantisation of energy - how at the atomic level energy comes wrapped up in bite-sized chunks - resolved a problem in a completely new area of physics. It was Walter Nernst, an eminent physicist from Berlin, who made others sit up and take note as they discovered that he had been to see Einstein in Zurich. Soon it was clear why. Nernst had succeeded in accurately measuring the specific heats of solids at low temperatures and found the results to be in total agreement with Einstein's predictions based on his quantum solution.
With each passing success his reputation soared ever higher, and Einstein was offered an ordinary professorship at the German University in Prague. It was an opportunity he could not refuse, even if it meant leaving Switzerland after fifteen years. Einstein, Mileva and their sons Hans Albert and Eduard, who was not yet one, moved to Prague in April 1911.
'I no longer ask whether these quanta really exist', Einstein wrote to his friend Michele Besso soon after taking up his new post. 'Nor do I try to construct them any longer, for I now know that my brain cannot get through in this way.' Instead, he told Besso, he would limit himself to trying to understand the consequences of the quantum.85 There were others who also wanted to try. Less than a month later, on 9 June, Einstein received a letter and an invitation from an unlikely correspondent. Ernst Solvay, a Belgian industrialist who had made a substantial fortune by revolutionising the manufacture of sodium carbonate, offered to pay 1,000 francs to cover his travel expenses if he agreed to attend a week-long 'Scientific Congress' to be held in Brussels later that year from 29 October to 4 November.86 He would be one of a select group of 22 physicists from across Europe brought together to discuss 'current questions concerning the molecular and kinetic theories'. Planck, Rubens, Wien and Nernst would be attending. It was a summit meeting on the quantum.
Planck and Einstein were among the eight asked to prepare reports on a particular topic. To be written in French, German or English, they were to be sent out to the participants before the meeting and serve as the starting point for discussion during the planned sessions. Planck would discuss blackbody radiation theory, while Einstein had been assigned his quantum theory of specific heat. Although Einstein was accorded the honour of giving the final talk, a discussion of his quantum theory of light was not on the agenda.
'I find the whole undertaking extremely attractive,' Einstein wrote to Walter Nernst, 'and there is little doubt in my mind that you are its heart and soul.'87 By 1910 Nernst believed that the time was ripe to get to grips with the quantum that he regarded as nothing more than a 'rule with most curious, indeed grotesque properties'.88 He convinced Solvay to finance the conference and the Belgian spared no expense booking the plush Hotel Metropole as the venue. In its luxurious surroundings, with all their needs catered for, Einstein and his colleagues spent five days talking about the quantum. Whatever slim hopes he harboured for progress at what he called 'the Witches' Sabbath', Einstein returned to Prague disappointed and complained of learning nothing that he did not know before.89
Nevertheless, he had enjoyed getting to know some of the other 'witches'. Marie Curie, whom he found to be 'unpretentious', appreciated 'the clearness of his mind, the shrewdness with which he marshalled his facts and the depth of his knowledge'.90 During the congress it was announced that she had been awarded the Nobel Prize for chemistry. She had become the first scientist to win two, having already won the physics prize in 1903. It was a tremendous achievement that was overshadowed by the scandal that broke around her during the congress. The French press had learned that she was having an affair with a married French physicist. Paul Langevin, a slender man with an elegant moustache, was a delegate at the conference and the papers were full of stories that the pair had eloped. Einstein, who had seen no signs of a special relationship between the two, dismissed the reports as rubbish. Despite her 'sparkling intelligence', he thought Curie was 'not attractive enough to represent a danger to anyone'.91
Even though at times he appeared to waver under the strain, Einstein had been the first to learn to live with the quantum, and by doing so revealed a hidden element of the true nature of light. Another young theorist also learned to live with the quantum after he used it to resurrect a flawed and neglected model of the atom.