Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality - Manjit Kumar (2009)
Part III. TITANS CLASH OVER REALITY
'There is no quantum world. There is only an abstract quantum mechanical description.'
— NIELS BOHR
'I still believe in the possibility of a model of reality - that is to say, of a theory that represents things themselves and not merely the probability of their occurrence.'
— ALBERT EINSTEIN
Chapter 11. SOLVAY 1927
'Now, I am able to write to Einstein', Hendrik Lorentz wrote on 2 April 1926.1 Earlier that day this elder statesman of physics had been granted a private audience with the King of the Belgians. Lorentz had sought and received royal approval for Einstein's election to the scientific committee of the International Institute of Physics set up by industrialist Ernest Solvay. Once described by Einstein as 'a marvel of intelligence and exquisite tact', Lorentz had also obtained the king's permission to invite German physicists to the fifth Solvay conference scheduled for October 1927.2
'His Majesty expressed the opinion that, seven years after the war, the feelings which they aroused should be gradually damped down, that a better understanding between peoples was absolutely necessary for the future, and that science could help to bring this about', reported Lorentz.3 Aware that Germany's brutal violation of Belgian neutrality in 1914 was still fresh in the memory, the king felt 'it necessary to stress that in view of all that the Germans had done for physics, it would be very difficult to pass them over'.4 But passed over and isolated from the international scientific community they had been ever since the end of the war.
'The only German invited is Einstein who is considered for this purpose to be international', Rutherford told a colleague before the third Solvay conference in April 1921.5 Einstein decided not to attend because Germans were excluded, and instead went on a lecture tour of America to raise funds for the founding of the Hebrew University in Jerusalem. Two years later he said he would decline any invitation to the fourth Solvay conference because of the continuing prohibition on German participation. 'In my opinion it is not right to bring politics into scientific matters,' he wrote to Lorentz, 'nor should individuals be held responsible for the government of the country to which they happen to belong.'6
Unable to attend the 1921 conference because of ill health, Bohr too declined an invitation to Solvay 1924. He feared that to go might be interpreted by some as tacit approval of the policy to exclude the Germans. When Lorentz became president of the League of Nations' Committee on Intellectual Cooperation in 1925, he saw little prospect of the ban on German scientists from international conferences being lifted in the near future.7 Then, unexpectedly in October that same year, the door barring them was unlocked if not yet opened.
In an elegant palazzo in the small Swiss resort of Locarno, on the northern tip of Lake Maggiore, treaties were ratified that many hoped would ensure the future peace of Europe. Locarno was the sunniest place in Switzerland and an apt setting for such optimism.8 It had taken months of intense diplomatic negotiations to arrange the meeting so that emissaries of Germany, France and Belgium could settle their post-war borders with one another. The Locarno treaties paved the way for Germany's acceptance, in September 1926, into the League of Nations, and membership brought with it an end to the exclusion of her scientists from the international stage. When the King of Belgium gave his consent, prior to the final moves on the diplomatic chessboard, Lorentz wrote to Einstein asking him attend the fifth Solvay conference and to accept his election to the committee responsible for planning it. Einstein agreed, and in the coming months the participants were selected, the agenda finalised, and the coveted invitations sent out.
All those invited fell into one of three groups. The first were members of the scientific committee: Hendrik Lorentz (president), Martin Knudsen (secretary), Marie Curie, Charles-Eugène Guye, Paul Langevin, Owen Richardson and Albert Einstein.9 The second group consisted of a scientific secretary, a Solvay family representative, and three professors from the Free University of Brussels, invited as a matter of courtesy. The American physicist Irving Langmuir, due to visit Europe at the time, would be present as a guest of the committee.
The invitation made clear that the 'conference will be devoted to the new quantum mechanics and to questions connected with it'.10 This was reflected in the composition of the third group: Niels Bohr, Max Born, William L. Bragg, Léon Brillouin, Arthur H. Compton, Louis de Broglie, Pieter Debye, Paul Dirac, Paul Ehrenfest, Ralph Fowler, Werner Heisenberg, Hendrik Kramers, Wolfgang Pauli, Max Planck, Erwin Schrödinger and C.T.R. Wilson.
The old masters of quantum theory and the young turks of quantum mechanics would all travel to Brussels. Sommerfeld and Jordan were the most prominent of those not invited to what looked like the physicists' equivalent of a theological council convened to settle some disputed point of doctrine. During the conference, five reports would be presented: William L. Bragg on the intensity of X-ray reflection; Arthur Compton on disagreements between experiment and the electromagnetic theory of radiation; Louis de Broglie on the new dynamics of quanta; Max Born and Werner Heisenberg on quantum mechanics; and Erwin Schrödinger on wave mechanics. The last two sessions of the conference would be devoted to a wide-ranging general discussion concerning quantum mechanics.
Two names were missing from the agenda. Einstein had been asked, but decided he was 'not competent' enough to present a report. 'The reason,' he told Lorentz, 'is that I have not been able to participate as intensively in the modern development of quantum theory as would be necessary for that purpose. This is in part because I have on the whole too little receptive talent for fully following the stormy developments, in part also because I do not approve of the purely statistical way of thinking on which the new theory is founded.'11 It was not an easy decision, since Einstein had wanted to 'contribute something of value in Brussels', but he confessed: 'I have now given up that hope.'12
In fact Einstein had closely monitored 'the stormy developments' of the new physics, and indirectly stimulated and encouraged the work of de Broglie and Schrödinger. However, from the very beginning he doubted that quantum mechanics was a consistent and complete description of reality. Bohr's name was also missing. He too had played no direct part in the theoretical development of quantum mechanics, but had exerted his influence through discussions with the likes of Heisenberg, Pauli and Dirac who did.
All those invited to the fifth Solvay conference on 'Electrons and Photons' knew it was designed to address the most pressing problem of the day, more philosophy than physics: the meaning of quantum mechanics. What did the new physics reveal about the nature of reality? Bohr believed he had found the answer. For many he arrived in Brussels as king of the quantum, but Einstein was the pope of physics. Bohr was anxious 'to learn his reaction to the latest stage of the development which, to our view, went far in clarifying the problems which he had himself from the outset elicited so ingeniously'.13 What Einstein thought mattered deeply to Bohr.
So it was in a mood of great expectancy that most of the world's leading quantum physicists assembled at 10am on a grey, overcast Monday on 24 October 1927, at the Institute of Physiology in Léopold Park for the start of the first session. The conference had taken eighteen months to arrange and required the consent of a king and the ending of Germany's pariah status.
![]()
After a few brief words of welcome from Lorentz as president of the scientific committee and chair of the conference, the task of opening the proceedings fell to William L. Bragg, professor of physics at Manchester University. Now 37, Bragg was only 25 when he was awarded the Nobel Prize for physics in 1915, together with his father, William H. Bragg, for pioneering the use of X-rays to investigate the structure of crystals. He was the obvious choice to report on the latest data concerning the reflection of X-rays by crystals and how these results led to a better understanding of atomic structure. After Bragg's presentation, Lorentz invited questions and contributions from the floor. The agenda had been organised to allow ample time after each report for a thorough discussion. With Lorentz using his command of English, German and French to help those less fluent, Bragg, Heisenberg, Dirac, Born, de Broglie, and the old Dutch master himself were among those who took part in the discussion before the first session came to an end and everyone adjourned for lunch.
In the afternoon session, the American Arthur Compton reported on the failure of the electromagnetic theory of radiation to explain either the photoelectric effect or the increase in the wavelength of X-rays when they are scattered by electrons. Although awarded a share of the 1927 Nobel Prize only a few weeks earlier, genuine modesty prevented him from referring to this last phenomenon as the Compton effect, as it was universally known. Where James Clerk Maxwell's great nineteenth-century theory failed, Einstein's light-quantum, newly rebranded as the 'photon', succeeded in uniting theory and experiment. The reports presented by Bragg and Compton were intended to facilitate the discussion of theoretical concepts. At the end of the first day all the leading players had spoken bar one, Einstein.
After a leisurely reception on Tuesday morning at the Free University of Brussels, everyone reconvened in the afternoon to hear Louis de Broglie's paper on 'The new dynamics of quanta'. Speaking in French, de Broglie began by outlining his own contribution, the extension of wave-particle duality to matter, and how Schrödinger ingeniously developed it into wave mechanics. Then, treading carefully by conceding that Born's idea contained a great deal of truth, he offered an alternative to the probabilistic interpretation of Schrödinger's wave function.
In the 'pilot wave theory', as de Broglie later called it, an electron really exists both as a particle and a wave, in contrast to the Copenhagen interpretation where an electron behaves like either a particle or a wave depending on the type of experiment performed. Both particles and waves exist simultaneously, de Broglie argued, with the particle, akin to a surfer, riding a wave. The waves leading or 'piloting' the particles from one place to another were physically real rather than Born's abstract waves of probability. With Bohr and his associates determined to assert the primacy of the Copenhagen interpretation and Schrödinger still doggedly wanting to promote his views on wave mechanics, de Broglie's pilot wave proposal came under attack. Looking for support from the one man who might sway the neutrals, de Broglie was disappointed when Einstein remained silent.
On Wednesday, 26 October, the proponents of the two rival versions of quantum mechanics addressed the conference. During the morning session, Heisenberg and Born gave a joint report. It was divided into four broad sections: the mathematical formalism; the physical interpretation; the uncertainty principle; and the applications of quantum mechanics.
The presentation, like the writing of the report, was a double act. Born, the senior man, delivered the introduction and sections I and II before handing over to Heisenberg. 'Quantum mechanics,' they began, 'is based on the intuition that the essential difference between atomic physics and classical physics is the occurrence of discontinuities.'14 Then came the metaphorical tipping of their hats to colleagues sitting only feet away as they pointed out that quantum mechanics was essentially 'a direct continuation of the quantum theory founded by Planck, Einstein, and Bohr'.15
After an exposition of matrix mechanics, the Dirac-Jordan transformation theory, and the probability interpretation, they turned to the uncertainty principle and the 'actual meaning of Planck's constant h'.16 It was nothing less, they maintained, than the 'universal measure of the indeterminacy that enters the laws of nature through the dualism of waves and corpuscles'. In effect, if there were no wave-particle duality of matter and radiation there would be no Planck's constant and no quantum mechanics. In conclusion, they made the provocative statement that 'we consider quantum mechanics to be a closed theory, whose fundamental physical and mathematical assumptions are no longer susceptible of any modification'.17
Closure implied that no future developments would ever alter any of the fundamental features of the theory. Any such claim to the completeness and finality of quantum mechanics was something that Einstein could not accept. For him quantum mechanics was indeed an impressive achievement but not yet the real thing. Refusing to take the bait, Einstein took no part in the discussion that followed the report. Nor did any one else raise objections, as only Born, Dirac, Lorentz and Bohr spoke.
Paul Ehrenfest, sensing Einstein's disbelief at the boldness of the Born-Heisenberg assertion that quantum mechanics was a closed theory, scribbled a note and passed it to him: 'Don't laugh! There is a special section in purgatory for professors of quantum theory, where they will be obliged to listen to lectures on classical physics ten hours every day.'18 'I laugh only at their naiveté', Einstein replied. 'Who knows who would have the [last] laugh in a few years?'
After lunch it was Schrödinger who took centre stage as he delivered his report in English on wave mechanics. 'Under this name at present two theories are being carried on, which are indeed closely related but not identical', he said.19 There was really only one theory, but it was effectively split in two. One part concerned waves in ordinary, everyday three-dimensional space, while the other required a highly abstract multi-dimensional space. The problem, Schrödinger explained, was that for anything other than a moving electron this was a wave that existed in a space with more than three dimensions. Whereas the single electron of the hydrogen atom could be accommodated in a three-dimensional space, helium with two electrons needed six dimensions. Nevertheless, Schrödinger argued that this multi-dimensional space, known as configuration space, was only a mathematical tool and ultimately whatever was being described, be it many electrons colliding or orbiting the nucleus of an atom, the process took place in space and time. 'In truth, however, a complete unification of the two conceptions has not yet been achieved', he admitted, before going on to outline both.20
Although physicists found it easier to use wave mechanics, no leading theorist agreed with Schrödinger's interpretation of the wave function of a particle as representing the cloud-like distribution of its charge and mass. Undeterred by the widespread acceptance of Born's alternative probability interpretation, Schrödinger highlighted his own and questioned the accepted notion of the 'quantum jump'.
From the moment he received the invitation to speak in Brussels, Schrödinger was acutely aware of the possibility of a clash with the 'matricians'. The discussion began with Bohr asking if a remark about 'difficulties' later in Schrödinger's report implied that a result he had stated earlier was incorrect. Schrödinger dealt with Bohr's inquiry comfortably, only to find Born challenging the correctness of another calculation. Somewhat annoyed, he said it was 'perfectly correct and rigorous and that this objection by Mr Born is unfounded'.21
After a couple of others had spoken, it was Heisenberg's turn: 'Mr Schrödinger says at the end of his report that the discussion he has given reinforces the hope that when our knowledge will be deeper it will be possible to explain and to understand in three dimensions the results provided by the multi-dimensional theory. I see nothing in Mr Schrödinger's calculations that would justify this hope.'22 Schrödinger argued that his 'hope of achieving a three-dimensional conception is not quite utopian'.23 A few minutes later the discussion ended and brought to a close the first part of the proceedings, the presentation of the commissioned reports.
When it was already too late to change the dates, it was discovered that the Académie des Sciences in Paris had chosen Thursday, 27 October to mark the centenary of the death of the French physicist Augustin Fresnel. It was decided that the Solvay meeting would be suspended for a day and a half to allow those wishing to attend the ceremonial event to do so and return for the climax of the conference, a wide-ranging general discussion spread over the last two sessions. Lorentz, Einstein, Bohr, Born, Pauli, Heisenberg and de Broglie were among the twenty who travelled to Paris to honour a kindred spirit.
![]()
Amid the distraction of German, French and English voices all seeking permission from Lorentz to speak next, Paul Ehrenfest suddenly got up and walked over to the blackboard and wrote: 'The Lord did there confound the languages of all the earth.' As he returned to his chair there was laughter as his colleagues realised that Ehrenfest was not just referring to the biblical Tower of Babel. The first session of the general discussion began on Friday afternoon, 28 October, with Lorentz making some introductory remarks as he tried to focus minds on the issues of causality, determinism, and probability. Were quantum events caused or not? Or as he put it: 'Could one not maintain determinism by making it an article of faith? Must one necessarily elevate indeterminism to a principle?'24 Offering no further thoughts of his own, Lorentz invited Bohr to address the meeting. As he spoke about the 'epistemological problems confronting us in quantum physics', it was clear to all present that Bohr was attempting to convince Einstein about the correctness of the Copenhagen solutions.25
When the conference proceedings were published in French in December 1928, many mistook Bohr's contribution, then and later, as one of the official reports. When asked for an edited version of his comments for inclusion, Bohr requested that a much-expanded version of his Como lecture, which had been published the previous April, be reprinted in lieu of his remarks. Bohr being Bohr, his request was granted.26
Einstein listened as Bohr outlined his belief that wave-particle duality was an intrinsic feature of nature that was explicable only within the framework of complementarity, that complementarity underpinned the uncertainty principle which exposed the limits of applicability of classical concepts. However, the ability to communicate unambiguously the results of experiments probing the quantum world, Bohr explained, required the experimental set-up as well as the observations themselves to be expressed in a language 'suitably refined by the vocabulary of classical physics'.27
In February 1927, as Bohr was edging towards complementarity, Einstein had given a lecture in Berlin on the nature of light. He argued that instead of either a quantum or a wave theory of light, what was needed was 'a synthesis of both conceptions'.28 It was a view he had first expressed almost twenty years earlier. Where he had long hoped to see some sort of 'synthesis', Einstein now heard Bohr imposing segregation through complementarity. It was either waves or particles depending on the choice of experiment.
Scientists had always conducted their experiments on the unspoken assumption that they were passive observers of nature, able to look without disturbing what they were looking at. There was a razor-sharp distinction between object and subject, between the observer and observed. According to the Copenhagen interpretation, this was not true in the atomic realm, as Bohr identified what he called the 'essence' of the new physics - the 'quantum postulate'.29It was a term he introduced to capture the existence of discontinuity in nature due to indivisibility of the quantum. The quantum postulate, said Bohr, led to no clear separation of the observer and the observed. When investigating atomic phenomena, the interaction between what is measured and the measuring equipment meant, according to Bohr, that 'an independent reality in the ordinary physical sense can neither be ascribed to the phenomenon nor to the agencies of observation'.30
The reality Bohr envisaged did not exist in the absence of observation. According to the Copenhagen interpretation, a microphysical object has no intrinsic properties. An electron simply does not exist at any place until an observation or measurement is performed to locate it. It does not have a velocity or any other physical attribute until it is measured. In between measurements it is meaningless to ask what is the position or velocity of an electron. Since quantum mechanics says nothing about a physical reality that exists independently of the measuring equipment, only in the act of measurement does the electron become 'real'. An unobserved electron does not exist.
'It is wrong to think that the task of physics is to find out how nature is', Bohr would argue later.31 'Physics concerns what we can say about nature.' Nothing more. He believed that science had but two goals, 'to extend the range of our experience and to reduce it to order'.32 'What we call science,' Einstein once said, 'has the sole purpose of determining what is.'33 Physics for him was an attempt to grasp reality, as it is, independent of observation. It is in this sense, he said, that 'one speaks of "physical reality"'.34 Bohr, armed with the Copenhagen interpretation, was not interested in what 'is', but in what we can say to each other about the world. As Heisenberg later stated, unlike objects in the everyday world, 'atoms or the elementary particles themselves are not as real; they form a world of potentialities or possibilities rather than one of things or facts'.35
For Bohr and Heisenberg, the transition from the 'possible' to the 'actual' took place during the act of observation. There was no underlying quantum reality that exists independently of the observer. For Einstein, a belief in the existence of an observer-independent reality was fundamental to the pursuit of science. At stake in the debate that was about to begin between Einstein and Bohr was the soul of physics and the nature of reality.
![]()
After Bohr's contribution, three others had already spoken when Einstein indicated to Lorentz that he wanted to break his self-imposed silence. 'Despite being conscious of the fact that I have not entered deeply enough into the essence of quantum mechanics,' he said, 'nevertheless I want to present here some general remarks.'36 Quantum mechanics, Bohr had argued, 'exhausted the possibilities of accounting for observable phenomena'.37 Einstein disagreed. A line had been drawn in the microphysical sands of the quantum realm. Einstein knew that the onus was on him to show that the Copenhagen interpretation was inconsistent and thereby wreck the claims of Bohr and his supporters that quantum mechanics was a closed and complete theory. He resorted to his favourite tactic - the hypothetical thought experiment conducted in the laboratory of the mind.
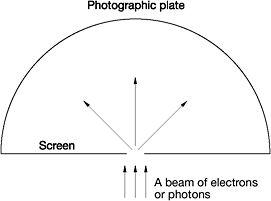
Figure 13: Einstein's single-slit thought experiment
Einstein went over to the blackboard and drew a line representing an opaque screen with a small slit in it. Just behind the screen he drew a semicircular curve representing a photographic plate. Using the sketch, Einstein outlined his experiment. When a beam of electrons or photons strikes the screen, some will pass through the slit and hit the photographic plate. Because of the narrowness of the slit, the electrons passing through it will diffract like waves in every possible direction. In keeping with the demands of quantum theory, Einstein explained, the electrons travelling outwards from the slit towards the photographic plate do so as spherical waves. Nonetheless, the electrons actually strike the plate as individual particles. There were, said Einstein, two distinct points of view concerning this thought experiment.
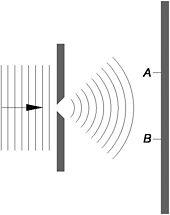
Figure 14: A later rendition by Bohr of Einstein's single-slit thought experiment
According to the Copenhagen interpretation, before any observation is made, and striking the photographic plate counts as such, there is a non-zero probability of detecting an individual electron at every point on the plate. Even though the wave-like electron is spread over a large region of space, the very moment a particular electron is detected at point A, the probability of finding it at point B or anywhere else on the plate instantly becomes zero. Since the Copenhagen interpretation maintains that quantum mechanics gives a complete description of individual electron events in the experiment, the behaviour of each electron is described by a wave function.
Here's the rub, said Einstein. If prior to the observation the probability of finding the electron was 'smeared' over the entire photographic plate, then the probability at B and everywhere else had to be instantaneously affected at the very moment the electron hit the plate at point A. Such an instantaneous 'collapse of the wave function' implied the propagation of some sort of faster-than-light cause and effect outlawed by his special theory of relativity. If an event at A is the cause of another at B, then there must be a time lapse between them to allow a signal to travel at light speed from A to B. Einstein believed the violation of this requirement, later called locality, indicated that the Copenhagen interpretation was inconsistent and quantum mechanics was not a complete theory of individual processes. Einstein proposed an alternative explanation.
Each electron that passes through the slit follows one of many possible trajectories until it hits the photographic plate. However, the spherical waves do not correspond to individual electrons, argued Einstein, but to 'a cloud of electrons'.38 Quantum mechanics does not give any information about individual processes, but only about what he called an 'ensemble' of processes.39 Though each individual electron of the ensemble follows its own distinct trajectory from slit to plate, the wave function does not represent an individual electron but the electron cloud. Therefore, the square of the wave function, |![]() (A)|2, represents not the probability of finding a particular electron at A, but that of finding any member of the ensemble at that point.40 It was, Einstein said, a 'purely statistical' interpretation, by which he meant that the statistical distribution of the large number of electrons striking the plate produced the characteristic diffraction pattern.41
(A)|2, represents not the probability of finding a particular electron at A, but that of finding any member of the ensemble at that point.40 It was, Einstein said, a 'purely statistical' interpretation, by which he meant that the statistical distribution of the large number of electrons striking the plate produced the characteristic diffraction pattern.41
Bohr, Heisenberg, Pauli and Born were not entirely sure what Einstein was driving at. He had not clearly stated his aim: to show that quantum mechanics was inconsistent and therefore an incomplete theory. Sure, the wave function collapses instantaneously, they thought, but it was an abstract wave of probability, not a real wave travelling in ordinary three-dimensional space. Nor was it possible to choose between the two viewpoint Einstein outlined on the basis of observing what happens to an individual electron. In both cases an electron passes through the slit and hits the plate at some point.
'I feel myself in a very difficult position because I don't understand what precisely is the point which Einstein wants to [make]', said Bohr.42 'No doubt it is my fault.' Remarkably, he then said: 'I do not know what quantum mechanics is. I think we are dealing with some mathematical methods which are adequate for [a] description of our experiments.'43 Instead of responding to Einstein's analysis, Bohr simply went on to restate his own views. But in this game of quantum chess, the Danish grandmaster later recounted in a paper, written in 1949 to celebrate his opponent's 70th birthday, the reply he gave that evening and on the last day of the conference in 1927.44
According to Bohr, Einstein's analysis of his thought experiment tacitly assumed that the screen and photographic plate both had a well-defined position in space and time. However, maintained Bohr, this implied that both had an infinite mass, for only then would there be no uncertainty in either position or time as the electron emerged from the slit. As a result, the exact momentum and energy of the electron is unknown. This was the only possible scenario, argued Bohr, given that the uncertainty principle implies that the more precisely the electron's position is known, the more inexact any concurrent measurement of its momentum must be. The infinitely heavy screen in Einstein's imaginary experiment left no room for uncertainty in the space and time location of the electron at the slit. However, such precision came at a price: its momentum and energy were completely indeterminate.
It was more realistic, Bohr suggested, to assume that the screen did not have an infinite mass. Although still much heavier, the screen would now move when the electron passed through the slit. While any such movement would be so small as to be impossible to detect in the laboratory, its measurement presented no problem in the abstract world of the idealised thought experiment furnished, as it was, with measuring devices capable of perfect accuracy. Because the screen moves, the position of the electron in space and time is uncertain during the process of diffraction, resulting in a corresponding uncertainty in both its momentum and energy. However, compared to the case of an infinitely massive screen, it would lead to an improved prediction of where the diffracted electron will hit the photographic plate. Within the limits imposed by the uncertainty principle, argued Bohr, quantum mechanics was as complete a description of individual events as was possible.
Unimpressed by Bohr's reply, Einstein asked him to consider the possibility of controlling and measuring the transfer of momentum and energy between the screen and the particle, be it an electron or a photon, as it passed through the slit. Then, he argued, the state of the particle immediately afterwards could be determined with an accuracy greater than that allowed by the uncertainty principle. As the particle passes through the slit, said Einstein, it would be deflected and its trajectory towards the photographic plate would be determined by the law of conservation of momentum, which requires the sum total of the momenta of two bodies (particle and screen) that interact to remain constant. If the particle is deflected upwards, then the screen must be pushed downwards and vice versa.
Having used the moveable screen introduced by Bohr for his own ends, Einstein modified the imaginary experiment further by inserting a two-slit screen between the moveable screen and the photographic plate.
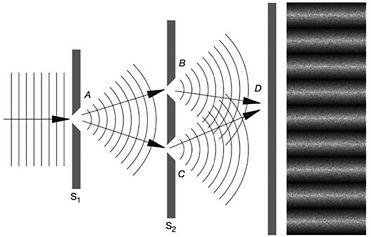
Figure 15: Einstein's two-slits thought experiment. At far right, the resulting interference pattern on the screen is shown
Einstein reduced the intensity of a beam until only one particle at a time passed through the slit in the first screen, S1, and one of the two slits of the second screen, S2, before hitting the photographic plate. As each particle left an indelible mark where it hit the plate, something remarkable would happen. What initially appeared to be a random sprinkling of specks was slowly transformed, as more and more particles left their imprint, by the laws of statistics into the characteristic interference pattern of light and dark bands. While each particle was responsible for only a single mark, it nevertheless contributed decisively through some statistical imperative to the overall interference pattern.
By controlling and measuring the transfer of momentum between the particle and the first screen it was possible, said Einstein, to determine if the particle was deflected towards the upper or lower slit in the second screen. From where it hit the photographic plate and the movement of the first screen, it was possible to trace through which of the two slits the particle had passed. It appeared that Einstein had devised an experiment in which it was possible to simultaneously determine the position and momentum of a particle with a greater precision than the uncertainty principle allowed. In the process he also seemed to have contradicted another fundamental tenet of the Copenhagen interpretation. Bohr's framework of complementarity posited that either particle-like or wave-like properties of an electron or a photon could be manifest in any given experiment.
There had to be a flaw in Einstein's argument, and Bohr set out to find it by sketching the sort of equipment needed to conduct the experiment. The apparatus he focused on was the first screen. Bohr realised that the control and measurement of the transfer of momentum between the particle and screen hinged on the screen's ability to move vertically. It is the observation of the screen moving either up or down as the particle passes through the slit that allows the determination of whether it passes through either the upper or lower slit in the second screen, after it strikes the photographic plate.
Einstein, despite his years at the Swiss Patent Office, had not considered the details of the experiment. Bohr knew that the quantum devil lay in the details. He replaced the first screen with one hanging by a pair of springs fixed to a supporting frame so that its vertical motion due to the transfer of momentum from a particle passing through the slit could be measured. The measuring device was simple: a pointer attached to the supporting frame and a scale engraved on the screen itself. It was crude, but sensitive enough to allow the observation of any individual interaction between screen and particle in an imaginary experiment.
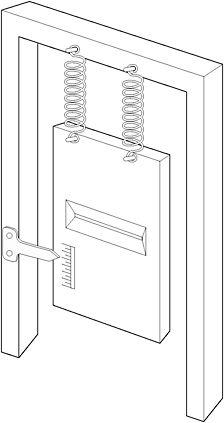
Figure 16: Bohr's design of a moveable first screen
Bohr argued that if the screen was already moving with an unknown velocity greater than any due to an interaction with a particle as it passed through the slit, then it would be impossible to ascertain the degree of momentum transfer and with it the trajectory of the particle. On the other hand, if it was possible to control and measure the transfer of momentum from particle to screen, the uncertainty principle implied a simultaneous uncertainty in the position of the screen and slit. However precise the measurement of the screen's vertical momentum, it was strictly matched, in accordance with the uncertainty principle, by a corresponding imprecision in the measurement of its vertical position.
Bohr went on to argue that the uncertainty in the position of the first screen destroys the interference pattern. For example, D on the photographic plate is a point of destructive interference, a dark spot in the interference pattern. A vertical displacement of the first screen would result in a change in the length of the two paths ABD and ACD. If the new lengths differed by half a wavelength, then instead of destructive interference there would be constructive interference and a bright spot at D.
To accommodate uncertainty in the vertical displacement of the first screen, S1, requires an 'averaging' over all of its possible positions. This leads to interference somewhere between the extremes of total constructive and total destructive interference, resulting in a washed-out pattern on the photographic plate. Controlling the transfer of momentum from the particle to the first screen allows the trajectory of the particle through a slit in the second screen to be tracked; however, it destroys the interference pattern, argued Bohr. He concluded that Einstein's 'suggested control of momentum transfer would involve a latitude in the knowledge of the position of the diaphragm [S1] which would exclude the appearance of the interference phenomena in question'.45 Bohr had not only defended the uncertainty principle but also the belief that the wave and particle aspects of a microphysical object cannot both appear in a single experiment, imaginary or not.
Bohr's rebuttal rested on the assumption that controlling and measuring the momentum transferred to S1 accurately enough to determine the particle's direction afterwards results in an uncertainty in the position of S1. The reason for this, Bohr explained, lay in reading the scale on S1. To do so, it has to be illuminated, and that requires the scattering of photons from the screen and results in an uncontrollable transfer of momentum. This impedes the precise measurement of the momentum transferred from the particle to the screen as it passes through the slit. The only way to eliminate the impact of the photon is by not illuminating the scale at all, making it impossible to read. Bohr had resorted to employing the same concept of 'disturbance' that he had earlier criticised Heisenberg for using as an explanation of the origin of uncertainty in the microscope thought experiment.
There was another curious phenomenon associated with the two-slit experiment. If one of the two slits has a shutter that is closed, then the interference pattern disappears. Interference occurs only when both slits are open at the same time. But how was that possible? A particle can go through only one slit. How did the particle 'know' that the other slit was open or closed?
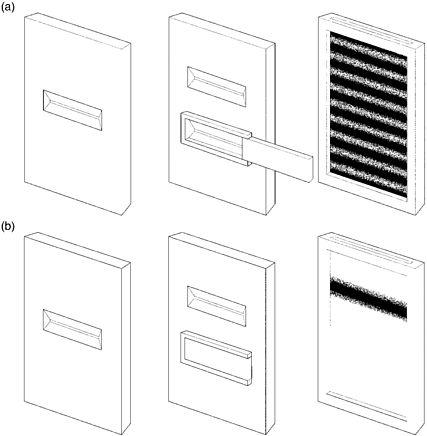
Figure 17: Two-slit experiment (a) with both slits open; (b) with one slit closed
Bohr had a ready answer. There was no such thing as a particle with a well-defined path. It was this lack of a definite trajectory that was behind the appearance of an interference pattern, even though it was particles, one at a time, which had passed through the two-slit set-up, and not waves. This quantum fuzziness enables a particle to 'sample' a variety of possible paths and so it 'knows' if one of the slits is open or closed. Whether it is open or not affects the particle's future path.
If detectors are placed in front of the two slits to sneak a look at which slit a particle is going to pass through, then it seems possible to close the other slit without affecting the particle's trajectory. When such a 'delayed-choice' experiment was later actually conducted, instead of an interference pattern there was an enlarged image of the slit. In trying to measure the position of the particle to establish through which slit it would pass, it is disturbed from its original course and the interference pattern fails to materialise.
The physicist has to choose, says Bohr, between 'either tracing the path of a particle or observing interference effects'.46 If one of the two slits of S2 is closed, then the physicist knows through which slit the particle passed before hitting the photographic plate, but there will be no interference pattern. Bohr argues that this choice allows an 'escape from the paradoxical necessity of concluding that the behaviour of an electron or a photon should depend on the presence of a slit in the diaphragm [S2] through which it could be proved not to pass'.47
The two-slit experiment was for Bohr 'a typical example' of the appearance of complementary phenomena under mutually exclusive experimental conditions.48 Given the quantum mechanical nature of reality, he argued, light was neither a particle nor a wave. It was both, and sometimes it behaved like a particle and sometimes like a wave. On any given occasion, nature's answer to whether it was a particle or a wave simply depended on the question asked - on the type of experiment performed. An experiment to determine through which slit in S2 a photon passed was a question that solicited a 'particle' answer and therefore no interference pattern. It was the loss of an independent, objective reality and not probability, God playing dice, that Einstein found unacceptable. Quantum mechanics, therefore, could not be the fundamental theory of nature that Bohr claimed it to be.
'Einstein's concern and criticism provided a most valuable incentive for us all to re-examine the various aspects of the situation as regards the description of atomic phenomena', recalled Bohr.49 A major point of contention, he stressed, was 'the distinction between the objects under investigation and the measuring instruments which serve to define, in classical terms, the conditions under which the phenomena appear'.50 In the Copenhagen interpretation the measuring instruments were inextricably linked with the object under investigation: no separation is possible.
While a microphysical object such as an electron was subject to the laws of quantum mechanics, the apparatus obeyed the laws of classical physics. Yet Bohr had to retreat in the face of Einstein's challenge as he applied the uncertainty principle to a macroscopic object, the first screen S1. By doing so, Bohr had imperiously consigned an element of the large-scale world of the everyday to the realm of the quantum as he failed to establish where is 'the cut' between the classical and the quantum worlds, the border between the macro and micro. It would not be the last time that Bohr played a questionable move in his game of quantum chess with Einstein. The spoils for the victor were just too high.
![]()
Einstein spoke only once more during the general discussion, when he asked a question. De Broglie recalled later that 'Einstein said hardly anything beyond presenting a very simple objection to the probability interpretation' and then 'he fell back into silence'.51 However, with all the participants staying at the Hotel Metropole, it was in its elegant art deco dining room that the keenest arguments took place, not in the conference room at the Institute of Physiology. 'Bohr and Einstein,' said Heisenberg, 'were in the thick of it all.'52
Surprisingly for an aristocrat, de Broglie spoke only French. He must have seen Einstein and Bohr deep in conversation in the dining room, with the likes of Heisenberg and Pauli listening closely. As they spoke in German, de Broglie did not realise that they were engaged in what Heisenberg called a 'duel'.53 The acknowledged master of the thought experiment, Einstein would arrive at breakfast armed with a new proposal that challenged the uncertainty principle and with it the much-lauded consistency of the Copenhagen interpretation.
The analysis would begin over coffee and croissants. It continued as Einstein and Bohr headed to the Institute of Physiology, usually with Heisenberg, Pauli and Ehrenfest trailing alongside. As they walked and talked, assumptions were probed and clarified before the start of the morning session. 'During the meeting and particularly in the pauses we younger people, mostly Pauli and I, tried to analyse Einstein's experiment,' Heisenberg said later, 'and at lunch time the discussions continued between Bohr and the others from Copenhagen.'54 Late in the afternoon, following further consultations among themselves, the collaborative effort would yield a rebuttal. During dinner back at the Metropole, Bohr would explain to Einstein why his latest thought experiment had failed to break the limits imposed by the uncertainty principle. Each time Einstein could find no fault with the Copenhagen response, but they knew, said Heisenberg, 'in his heart he was not convinced'.55
After several days, Heisenberg later recalled, 'Bohr, Pauli and I - knew that we could now be sure of our ground, and Einstein understood that the new interpretation of quantum mechanics cannot be refuted so simply'.56 But Einstein refused to yield. Even if it failed to capture the essence of his rejection of the Copenhagen interpretation, he would say, 'God does not play dice'. 'But still, it cannot be for us to tell God, how he is to run the world', replied Bohr on one occasion.57 'Einstein, I am ashamed of you,' said Paul Ehrenfest only half-joking, 'you are arguing against the new quantum theory just as your opponents argue about relativity theory.'58
The only impartial witness to the private encounters between Einstein and Bohr at Solvay 1927 was Ehrenfest. 'Einstein's attitude gave rise to ardent discussions within a small circle, in which Ehrenfest, who through the years had been a close friend of us both,' recalled Bohr, 'took part in a most active and helpful way.'59 A few days after the conference ended, Ehrenfest wrote a letter to his students at Leiden University vividly describing the goings-on in Brussels: 'Bohr towering completely over everybody. At first not understood at all (Born was also there), then step by step defeating everybody. Naturally once again the awful Bohr incantation terminology. (Poor Lorentz as interpreter between the British and the French who were absolutely unable to understand each other. Summarizing Bohr. And Bohr responding with polite despair.) Every night at 1 a.m. Bohr came into my room just to say ONE SINGLE WORD to me, until 3 a.m. It was delightful for me to be present during the conversations between Bohr and Einstein. Like a game of chess. Einstein all the time with new examples. … to break the UNCERTAINTY RELATION. Bohr from out of the philosophical smoke clouds constantly searching for the tools to crush one example after the other. Einstein like a jack-in-the-box, jumping out fresh every morning. Oh, that was priceless. But I am almost without reservation pro Bohr and contra Einstein.'60 However, Ehrenfest admitted 'that he would not be able to find relief in his own mind before concord with Einstein was reached'.61
At Solvay 1927 the discussions with Einstein were conducted, Bohr said later, in 'a most humorous spirit'.62 Yet he noted wistfully, 'a certain difference in attitude and outlook remained, since with his mastery for coordinating apparently contrasting experiences without abandoning continuity and causality, Einstein was perhaps more reluctant to renounce such ideals than someone for whom renunciation in this respect appeared to be the only way to proceed with the immediate task of coordinating the multifarious evidence regarding atomic phenomena, which accumulated from day to day in the exploration of this new field of knowledge.'63 It was Einstein's very successes, implied Bohr, that kept him anchored in the past.
![]()
The fifth Solvay conference ended with Bohr, in the minds of those gathered in Brussels, having successfully argued for the logical consistency of the Copenhagen interpretation, but failing to convince Einstein that it was the only possible interpretation of what was a 'complete', closed theory. On his journey home, Einstein travelled to Paris with a small group that included de Broglie. 'Carry on', he told the French prince as they parted company. 'You are on the right road.'64 But de Broglie, disheartened at the lack of support in Brussels, would soon recant and accept the Copenhagen interpretation. When Einstein reached Berlin he was exhausted and subdued. Within a fortnight he wrote to Arnold Sommerfeld that quantum mechanics 'may be a correct theory of the statistical laws, but it is an inadequate conception of individual elementary processes'.65
While Paul Langevin later said that 'the confusion of ideas reached its zenith' at Solvay 1927, for Heisenberg this meeting of minds was the decisive turning point in establishing the correctness of the Copenhagen interpretation.66'I am satisfied in every respect with the scientific results', he wrote as the conference ended.67 'Bohr's and my views have been generally accepted; at least serious objections are no longer being made, not even by Einstein and Schrödinger.' As far as Heisenberg was concerned, they had won. 'We could get anything clear by using the old words and limiting them by the uncertainty relations and still get a completely consistent picture', he recalled almost 40 years later. When asked whom he meant by 'we', Heisenberg replied: 'I could say that at that time it was practically Bohr, Pauli, and myself.'68
Bohr never used the term the 'Copenhagen interpretation', nor did anyone else until Heisenberg in 1955. Yet from a handful of adherents it quickly spread so that for most physicists the 'Copenhagen interpretation of quantum mechanics' became synonymous with quantum mechanics. Three factors lay behind this rapid dissemination and acceptance of the 'Copenhagen spirit'. The first was the pivotal role of Bohr and his institute. Inspired by his stay in Rutherford's laboratory in Manchester as a young postdoctoral student, Bohr had managed to create an institute of his own with the same zing in the air - the sense that anything was possible.
'Bohr's Institute quickly became the world centre of quantum physics, and to paraphrase the old Romans, "all roads lead to Blegdamsvej 17"', recalled the Russian George Gamov who arrived there in the summer of 1928.69 The Kaiser Wilhelm Institute of Theoretical Physics of which Einstein was the director existed only on paper, and he preferred it that way. While he usually worked alone, or later with an assistant who carried out the calculations, Bohr fathered many scientific children. The first to rise to prominence and positions of authority were Heisenberg, Pauli and Dirac. Though only young men, as Ralph Kronig later recalled, other young physicists did not dare to go against them. Kronig, for one, had not published the idea of electron spin after Pauli ridiculed it.
Secondly, around the time of Solvay 1927 a number of professorships became vacant. Those who had helped create the new physics filled nearly all of these. The institutes they headed quickly began to attract many of best and brightest students from Germany and across Europe. Schrödinger had secured the most prestigious position, as Planck's successor in Berlin. Immediately after the Solvay conference, Heisenberg arrived in Leipzig to take up his post as professor and director of the institute for theoretical physics. Within six months, in April 1928, Pauli moved from Hamburg to a professorship at the EHT in Zurich. Pascual Jordan, whose mathematical skills had been vital to the development of matrix mechanics, succeeded Pauli in Hamburg. Before long, through regular visits and the exchange of assistants and students between each other and Bohr's institute, Heisenberg and Pauli established Leipzig and Zurich as centres of quantum physics. With Kramers already installed at the University of Utrecht and Born at Göttingen, the Copenhagen interpretation soon became quantum dogma.
Lastly, despite their differences, Bohr and his younger associates always presented a united front against all challenges to the Copenhagen interpretation. The one exception was Paul Dirac. Appointed Lucasian Professor of Mathematics at Cambridge University in September 1932, a chair once occupied by Isaac Newton, Dirac was never interested in the question of interpretation. It seemed to him to be a pointless preoccupation that led to no new equations. Tellingly, he called himself a mathematical physicist, whereas neither his contemporaries Heisenberg and Pauli nor Einstein and Bohr ever described themselves as such. They were theoretical physicists to a man, as was Lorentz, the acknowledged elder statesman of the clan who died in February 1928. 'To me personally,' Einstein wrote later, 'he meant more than all the others encountered in my lifetime.'70
Soon Einstein's own health became a matter of concern. In April 1928 during a short visit to Switzerland he collapsed as he carried his suitcase up a steep hill. At first it was thought that he had suffered a heart attack, but then an enlargement of the heart was diagnosed. Later Einstein told his friend Michele Besso that he had felt 'close to croaking', before adding, 'which of course one shouldn't put off unduly'.71 Once back in Berlin under Elsa's watchful eye, visits by friends and colleagues were strictly rationed. She was once more Einstein's gatekeeper and nurse, as she had been after he had fallen ill following his Herculean effort in formulating the general theory of relativity. This time Elsa needed help and hired a friend's unmarried sister. Helen Dukas was 32 and became Einstein's trusted secretary and friend.72
As he recuperated, a paper by Bohr was published in three languages: English, German and French. The English version, entitled 'The Quantum Postulate and the Recent Development of Atomic Theory', appeared on 14April 1928. A footnote stated: 'The content of this paper is essentially the same as that of a lecture on the present state of quantum theory delivered on September 16, 1927, at the Volta celebrations in Como.'73 In truth, Bohr had produced a more refined and advanced exposition of his ideas surrounding complementarity and quantum mechanics than he had presented in either Como or Brussels.
Bohr sent a copy to Schrödinger, who replied: 'if you want to describe a system, e.g. a mass point by specifying its [momentum] p and [position] q, then you find that this description is only possible with a limited degree of accuracy.'74 What was therefore needed, Schrödinger argued, was the introduction of new concepts with respect to which this limitation no longer applies. 'However,' he concluded, 'it will no doubt be very difficult to invent this conceptual scheme, since - as you emphasize so impressively - the new-fashioning required touches upon the deepest levels of our experience: space, time and causality.'
Bohr wrote back thanking Schrödinger for his 'not altogether unsympathetic attitude', but he did not see the need for 'new concepts' in quantum theory since the old empirical concepts appeared inseparably linked to the 'foundations of the human means of visualization'.75 Bohr restated his position that it was not a question of a more or less arbitrary limitation in the applicability of the classical concepts, but an inescapable feature of complementarity that emerges in an analysis of the concept of observation. He ended by encouraging Schrödinger to discuss the contents of his letter with Planck and Einstein. When Schrödinger informed him of the exchange with Bohr, Einstein replied that the 'Heisenberg-Bohr tranquilizing philosophy - or religion? - is so delicately contrived that for the time being, it provides a gentle pillow for the true believer from which he cannot very easily be aroused. So let him lie there.'76
Four months after collapsing, Einstein was still weak but no longer confined to his bed. To continue his convalescence he rented a house in the sleepy town of Scharbeutz on the Baltic coast. There he read Spinoza and enjoyed being away from the 'idiotic existence one leads in the city'.77 It was almost a year before he was well enough to return to his office. He would work there all morning before going home for lunch and a rest until three o'clock. 'Otherwise he was always working,' recalled Helen Dukas, 'sometimes all through the night.'78
During the Easter vacation of 1929 Pauli went to see Einstein in Berlin. He found Einstein's 'attitude regarding modern quantum physics reactionary' because he continued to believe in a reality where natural phenomena unfolded according to the laws of nature, independently of an observer.79 Shortly after Pauli's visit, Einstein made his views perfectly clear as he received the Planck medal from Planck himself. 'I admire to the highest degree the achievements of the younger generation of physicists which goes by the name quantum mechanics and believe in the deep level of truth of that theory,' he told the audience, 'but I believe that the restriction to statistical laws will be a passing one.'80 Einstein had already embarked on his solitary journey in search of a unified field theory that he believed would save causality and an observer-independent reality. In the meantime he would continue to challenge what was becoming the quantum orthodoxy, the Copenhagen interpretation. When they met again in Brussels at the sixth Solvay conference in 1930, Einstein presented Bohr with an imaginary box of light.