Quantum: Einstein, Bohr and the Great Debate About the Nature of Reality - Manjit Kumar (2009)
Part II. BOY PHYSICS
Chapter 10. UNCERTAINTY IN COPENHAGEN
As Werner Heisenberg stood in front of the blackboard, with his notes spread out on the table before him, he was nervous. The brilliant 25-year-old physicist had every reason to be. It was Wednesday, 28 April 1926, and he was about to deliver a lecture on matrix mechanics to the famed physics colloquium at Berlin University. Whatever the merits of Munich or Göttingen, it was Berlin that Heisenberg rightly called 'the stronghold of physics in Germany'.1His eyes scanned the faces in the audience and settled on four men sitting in the front row, each with a Nobel Prize to his name: Max von Laue, Walter Nernst, Max Planck, and Albert Einstein.
Any nerves at this 'first chance to meet so many famous men' quickly subsided as Heisenberg, by his own reckoning, presented 'a clear account of the concepts and mathematical foundations of what was then a most unconventional theory'.2 As the audience drifted away after the lecture, Einstein invited Heisenberg back to his apartment. During the half-hour stroll to Haberlandstrasse, Einstein asked Heisenberg about his family, education and early research. It was only when they were comfortably seated in his apartment that the real conversation began, recalled Heisenberg, as Einstein probed 'the philosophical background of my recent work'.3 'You assume the existence of electrons inside the atom, and you are probably right to do so', said Einstein. 'But you refuse to consider their orbits, even though we can observe electron tracks in a cloud chamber. I should very much like to hear more about your reasons for making such strange assumptions.'4 This was just what he had hoped for, a chance to win over the 47-year-old quantum master.
'We cannot observe electron orbits inside the atom,' replied Heisenberg, 'but the radiation which an atom emits during discharges enables us to deduce the frequencies and corresponding amplitudes of its electrons.'5 Warming to his theme, he explained that 'since a good theory must be based on directly observable magnitudes, I thought it more fitting to restrict myself to these, treating them, as it were, as representatives of the electron orbits'.6 'But you don't seriously believe,' Einstein protested, 'that none but observable magnitudes must go into a physical theory?'7 It was a question that struck at the very foundations on which Heisenberg had constructed his new mechanics. 'Isn't that precisely what you have done with relativity?' he countered.
A 'good trick should not be tried twice', smiled Einstein.8 'Possibly I did use this kind of reasoning,' he conceded, 'but it is nonsense all the same.' Although it might be heuristically useful to bear in mind what one has actually observed, in principle, he argued, 'it is quite wrong to try founding a theory on observable magnitudes alone'. 'In reality the very opposite happens. It is the theory which decides what we can observe.'9 What did Einstein mean?
Almost a century before, in 1830, the French philosopher Auguste Comte had argued that, while every theory has to be based on observation, the mind also needs a theory in order to make observations. Einstein tried to explain that observation was a complex process, involving assumptions about phenomena that are used in theories. 'The phenomenon under observation produces certain events in our measuring apparatus', said Einstein.10 'As a result, further processes take place in the apparatus, which eventually and by complicated paths produce sense impressions and help fix the effects in our consciousness.' These effects, Einstein maintained, depend on our theories. 'And in your theory,' he told Heisenberg, 'you quite obviously assume that the whole mechanism of light transmission from the vibrating atom to the spectroscope or to the eye works just as one has always supposed it does, that is, essentially according to Maxwell's law. If that were no longer the case, you could not possibly observe any of the magnitudes you call observable.'11 Einstein continued to press: 'Your claim that you are introducing none but observable magnitudes is therefore an assumption about a property of the theory that you are trying to formulate.'12 'I was completely taken aback by Einstein's attitude, though I found his arguments convincing', Heisenberg later admitted.13
While Einstein was still a patent clerk he had studied the work of the Austrian physicist Ernst Mach, for whom the goal of science was not to discern the nature of reality, but to describe experimental data, the 'facts', as economically as possible. Every scientific concept was to be understood in terms of its operational definition - a specification of how it could be measured. It was while under the influence of this philosophy that Einstein had challenged the established concepts of absolute space and time. But he had long since abandoned Mach's approach because, as he told Heisenberg, it 'rather neglects the fact that the world really exists, that our sense impressions are based on something objective'.14
As he left the apartment disappointed at his failure to persuade Einstein, Heisenberg needed to make a decision. In three days' time, on 1 May, he was due in Copenhagen to begin his dual appointment as Bohr's assistant and as a lecturer at the university. However, he had just been offered an ordinary professorship at Leipzig University. Heisenberg knew it was a tremendous honour for one so young, but should he accept? Heisenberg told Einstein of the difficult choice he had to make. Go and work with Bohr, was his advice. The next day, Heisenberg wrote to his parents that he was turning down the Leipzig offer. 'If I continue to produce good papers,' he reassured himself and them, 'I will always receive another call; otherwise I don't deserve it.'15
![]()
'Heisenberg is now here and we are all very much occupied with discussions about the new development of the quantum theory and the great prospect it holds out', Bohr wrote to Rutherford in the middle of May 1926.16Heisenberg lived at the institute in a 'cosy little attic flat with slanting walls' and a view of Faelled Park.17 Bohr and his family had moved into the plush and spacious director's villa next door. Heisenberg was such a regular visitor that he soon felt 'half at home with the Bohrs'.18 The enlargement and renovation of the institute had taken far longer than expected and Bohr was exhausted. Sapped of energy, he suffered a severe case of flu. As Bohr spent the next two months recovering, Heisenberg successfully used wave mechanics to account for the line spectrum of helium.
Once Bohr was back to his old self, living next door to him was something of a mixed blessing. 'After 8 or 9 o'clock in the evening Bohr, all of a sudden, would come up to my room and say, "Heisenberg, what do you think about this problem?" And then we would start talking and talking and quite frequently we went on till twelve or one o'clock at night.'19 Or he would invite Heisenberg over to the villa for a chat that lasted long into the evening, fuelled by glasses of wine.
As well as working with Bohr, Heisenberg gave two lectures a week on theoretical physics at the university in Danish. He was not much older than his students, and one of them could barely believe 'he was so clever since he looked like a bright carpenter's apprentice just returned from technical school'.20 Heisenberg quickly adapted to the rhythm of life at the institute and with his new colleagues enjoyed sailing, horse riding, and walking tours at the weekends. But there was less and less time for such activities after Schrödinger's visit at the beginning of October 1926.
Schrödinger and Bohr had failed to reach any sort of accord over the physical interpretation of either matrix or wave mechanics. Heisenberg saw how 'terribly anxious' Bohr was 'to get to the bottom of things'.21 In the months that followed, the interpretation of quantum mechanics was all that Bohr and his young apprentice talked about as they tried to reconcile theory and experiment. 'Bohr often came up to my room late at night to talk to me of the difficulties in quantum theory which tortured both of us', Heisenberg said later.22 Nothing caused them more pain than wave-particle duality. As Einstein told Ehrenfest: 'On the one hand waves, on the other quanta! The reality of both is firm as a rock. But the devil makes a verse out of this (which really rhymes).'23
In classical physics something can be either a particle or a wave; it cannot be both. Heisenberg had used particles and Schrödinger waves as they discovered their respective versions of quantum mechanics. Even the demonstration that both matrix and wave mechanics were mathematically equivalent had not yielded any deeper understanding of wave-particle duality. The crux of the whole problem, Heisenberg said, was that no one could answer the questions: 'Is an electron now a wave or is it a particle, and how does it behave if I do this and that and so on?'24 The harder Bohr and Heisenberg thought about wave-particle duality, the worse things seemed to become. 'Like a chemist who tries to concentrate his poison more and more from some kind of solution,' remembered Heisenberg, 'we tried to concentrate the poison of the paradox.'25 As they did so there was an increasing tension between the two men, as each adopted a different approach in an attempt to resolve the difficulties.
In the search for a physical interpretation of quantum mechanics, what the theory revealed about the nature of reality at the atomic level, Heisenberg was totally committed to particles, quantum jumps, and discontinuity. For him the particle aspect was dominant in wave-particle duality. He was not prepared to make room to accommodate anything remotely linked to Schrödinger's interpretation. To Heisenberg's horror, Bohr wanted to 'play with both schemes'.26 Unlike the young German, he was not wedded to matrix mechanics and had never been enthralled by any mathematical formalism. While Heisenberg's first port of call was always the mathematics, Bohr weighed anchor and sought to understand the physics behind the mathematics. In probing quantum concepts such as wave-particle duality, he was more interested in grasping the physical content of an idea rather than the mathematics it came wrapped in. Bohr believed that a way had to be found to allow for the simultaneous existence of both particles and waves in any complete description of atomic processes. Reconciling these two contradictory concepts was for him the key that would open the door leading to a coherent physical interpretation of quantum mechanics.
Ever since Schrödinger's discovery of wave mechanics it was understood that there was one quantum theory too many. What was needed was a single formulation, especially given that the two were mathematically the same. It was Paul Dirac and Pascual Jordan, independently of each other, who came up with just such a formalism that autumn. Dirac, who had arrived in Copenhagen in September 1926 for a six-month stay, showed that matrix and wave mechanics were just special cases of an even more abstract formulation of quantum mechanics called transformation theory. All that was missing was a physical interpretation of the theory, and the search for it was beginning to take its toll.
'Since our talks often continued till long after midnight and did not produce a satisfactory conclusion despite protracted efforts over several months,' recalled Heisenberg, 'both of us became utterly exhausted and rather tense.'27Bohr decided that enough was enough and went on a four-week skiing holiday in Guldbrandsdalen, Norway in February 1927. Heisenberg was glad to see him go, so that he 'could think about these hopelessly complicated problems undisturbed'.28 None was more pressing than the trajectory of an electron in a cloud chamber.
When Bohr met Rutherford at the research students' Christmas party in Cambridge in 1911, he was struck by the New Zealander's generous praise for the recent invention of the cloud chamber by C.T.R. Wilson. The Scotsman had managed to create clouds in a small glass chamber that contained air saturated with water vapour. Cooling the air by allowing it to expand caused the vapour to condense into minuscule water droplets on particles of dust, producing a cloud. Before long, Wilson was able to create a 'cloud' even after removing all traces of dust from the chamber. The only explanation he could offer was that the cloud was formed by condensation on ions present in the air within the chamber. However, there was another possibility. Radiation passing through the chamber could rip electrons from atoms in the air, forming ions, thereby leaving a trail of tiny water droplets in its wake. It was soon discovered that radiation did exactly that. Wilson appeared to have given physicists a tool for observing the trajectories of alpha and beta particles emitted from radioactive substances.
Particles followed well-defined paths, while waves, because they spread out, did not. However, quantum mechanics did not allow for the existence of the particle trajectories that were clearly visible for all to see in a cloud chamber. The problem seemed insurmountable. But it ought to be possible, Heisenberg was convinced, to establish a connection between what was observed in the cloud chamber and quantum theory, 'hard though it appeared to be'.29
Working late one evening in his small attic flat at the institute, Heisenberg's mind began to wander as he pondered the riddle of electron tracks in a cloud chamber where matrix mechanics said there should be none. All of a sudden he heard the echo of Einstein's rebuke that 'it is the theory that decides what we can observe'.30 Convinced that he was on to something, Heisenberg needed to clear his head. Although it was well past midnight, he went for a walk in the neighbouring park.
Barely feeling the chill, he began to focus on the precise nature of the electron track left behind in a cloud chamber. 'We had always said so glibly that the path of the electron in the cloud chamber could be observed', he wrote later.31 'But perhaps what we really observed was something much less. Perhaps we merely saw a series of discrete and ill-defined spots through which the electron had passed. In fact, all we do see in the cloud chamber are individual water droplets which must certainly be much larger than the electron.'32 There was no continuous, unbroken path, Heisenberg believed. He and Bohr had been asking the wrong questions. The one to answer was: 'Can quantum mechanics represent the fact that an electron finds itself approximately in a given place and that it moves approximately with a given velocity?'
Hurrying back to his desk, Heisenberg began manipulating the equations he knew so well. Quantum mechanics apparently placed restrictions on what could be known and observed. But how did the theory decide what can and cannot be observed? The answer was the uncertainty principle.
Heisenberg had discovered that quantum mechanics forbids, at any given moment, the precise determination of both the position and the momentum of a particle. It is possible to measure exactly either where an electron is or how fast it is moving, but not both simultaneously. It was nature's price for knowing one of the two exactly. In a quantum dance of give-and-take, the more accurately one is measured the less accurately the other can be known or predicted. If he was right, then Heisenberg knew that it meant no experiment probing the atomic realm would ever succeed in overcoming the limits imposed by the uncertainty principle. It was, of course, impossible to 'prove' such a claim, but Heisenberg was certain it must be so, given that all processes involved in any such experiment 'had necessarily to satisfy the laws of quantum mechanics'.33
In the days that followed he tested the uncertainty principle, or as he preferred to call it, the indeterminacy principle. In the laboratory of the mind, he conducted one imaginary 'thought experiment' after another in which it might be possible to measure position and momentum simultaneously with an accuracy that the uncertainty principle said was impossible. As calculation after calculation revealed that the uncertainty principle had not been violated, one particular thought experiment convinced Heisenberg that he had successfully demonstrated that 'It is the theory which decides what we can and cannot observe'.
Heisenberg had once discussed with a friend the difficulties surrounding the concept of electron orbits. His friend had argued that it should, in principle, be possible to construct a microscope that allowed electron paths inside the atom to be observed. However, such an experiment was now ruled out because, according to Heisenberg, 'not even the best microscope could cross the limits set by the uncertainty principle'.34 All he had to do was prove it theoretically by trying to determine the exact position of a moving electron.
To 'see' an electron required a special kind of microscope. Ordinary microscopes use visible light to illuminate an object and then focus the reflected light into an image. The wavelengths of visible light are much larger than an electron and therefore could not be used to determine its exact position as they washed over it like waves over a pebble. What was required was a microscope that used gamma rays, 'light' of extremely short wavelength and high frequency, to pinpoint its position. Arthur Compton, in 1923, had investigated X-rays striking electrons and found conclusive evidence for the existence of Einstein's light-quanta. Heisenberg imagined that, like two billiard balls colliding, when a gamma ray photon hits the electron, it is scattered into the microscope as the electron recoils.
There is, however, a discontinuous shove rather than a smooth transition in the electron's momentum due to the impact of the gamma ray photon. Since the momentum that an object possesses is its mass multiplied by its velocity, any change in its velocity causes a corresponding change in its momentum.35 When the photon hits the electron it jolts its velocity. The only way to minimise the discontinuous change in the electron's momentum is by reducing the energy of the photon, thereby lessening the impact of the collision. To do so entails using light of a longer wavelength and lower frequency. However, such a switch in wavelength means that it is no longer possible to pin down the exact position of the electron. The more precisely the electron's position is measured, the more uncertain or imprecise any measurement of its momentum and vice versa.36
Heisenberg showed that if ![]() p and
p and ![]() q (where
q (where ![]() is the Greek letter delta) are the 'imprecision' or 'uncertainty' with which the momentum and the position are known, then
is the Greek letter delta) are the 'imprecision' or 'uncertainty' with which the momentum and the position are known, then ![]() p multiplied by
p multiplied by ![]() q is always greater than or equal to h/2
q is always greater than or equal to h/2![]() :
: ![]() p
p![]() q
q![]() h/2
h/2![]() , where h is Planck's constant.37 This was the mathematical form of the uncertainty principle or the 'imprecision in knowledge of simultaneous measurements' of position and momentum. Heisenberg also discovered another 'uncertainty relation' involving a different pair of so-called conjugate variables, energy and time. If
, where h is Planck's constant.37 This was the mathematical form of the uncertainty principle or the 'imprecision in knowledge of simultaneous measurements' of position and momentum. Heisenberg also discovered another 'uncertainty relation' involving a different pair of so-called conjugate variables, energy and time. If ![]() E and
E and ![]() t are the uncertainties with which the energy E of a system can be determined and the time t at which E is observed, then
t are the uncertainties with which the energy E of a system can be determined and the time t at which E is observed, then ![]() E
E![]() t
t![]() h/2
h/2![]() .
.
At first there were some who thought that the uncertainty principle was the result of the technological shortcomings of the equipment used in an experiment. If the equipment could be improved, they believed, then the uncertainty would disappear. This misunderstanding arose because of Heisenberg's use of thought experiments to draw out the significance of the uncertainty principle. However, thought experiments are imaginary experiments employing perfect equipment under ideal conditions. The uncertainty discovered by Heisenberg is an intrinsic feature of reality. There could be no improvement, he argued, on the limits set by the size of Planck's constant and enforced by the uncertainty relations on the precision of what is observable in the atomic world. Rather than 'uncertain' or 'indeterminate', 'unknowable' may have been a more apt description of his remarkable discovery.
Heisenberg believed it was the act of measuring the position of the electron that made the precise determination of its momentum at the same time impossible. The reason appeared, as far as he was concerned, to be straightforward. The electron is disturbed unpredictably when struck by the photon used to 'see it' in order to locate its position. It was this unavoidable disturbance during the act of measurement that Heisenberg identified as the origin of uncertainty.38
It was an explanation that he believed was supported by the fundamental equation of quantum mechanics: pq-qp=-ih/2![]() , where p and q are the momentum and position of a particle. It was the inherent uncertainty of nature that lay behind non-commutativity - the fact that p×q does not equal q×p. If an experiment to locate an electron were followed by one measuring its velocity (and therefore its momentum) they would give two precise values. Multiplying the two values together yields an answer A. However, repeating the experiments in reverse order, measuring the velocity first and then the position, would lead to a completely different result, B. In each case the first measurement caused a disturbance that affected the outcome of the second. If there had been no disturbance, which was different in each experiment, then p×q would be the same as q×p. As pq-qp would then equal zero, there would be no uncertainty and no quantum world.
, where p and q are the momentum and position of a particle. It was the inherent uncertainty of nature that lay behind non-commutativity - the fact that p×q does not equal q×p. If an experiment to locate an electron were followed by one measuring its velocity (and therefore its momentum) they would give two precise values. Multiplying the two values together yields an answer A. However, repeating the experiments in reverse order, measuring the velocity first and then the position, would lead to a completely different result, B. In each case the first measurement caused a disturbance that affected the outcome of the second. If there had been no disturbance, which was different in each experiment, then p×q would be the same as q×p. As pq-qp would then equal zero, there would be no uncertainty and no quantum world.
Heisenberg was delighted as he saw the pieces fit neatly together. His version of quantum mechanics was built out of matrices representing observables such as position and momentum that do not commute. Ever since he discovered the strange rule that made the order in which two arrays of numbers were multiplied an essential component of the mathematical scheme of his new mechanics, the physical reason why this was so had been shrouded in mystery. Now he had lifted the veil. It was, according to Heisenberg, 'only the uncertainty specified by ![]() p
p![]() q
q![]() h/2
h/2![]() ', that 'creates room for the validity of the relations' in pq-qp=-ih/2
', that 'creates room for the validity of the relations' in pq-qp=-ih/2![]() .39 It was uncertainty, he claimed, that 'makes possible this equation without requiring that the physical meaning of the quantities p and q be changed'.40
.39 It was uncertainty, he claimed, that 'makes possible this equation without requiring that the physical meaning of the quantities p and q be changed'.40
The uncertainty principle had exposed a deep fundamental difference between quantum and classical mechanics. In classical physics both the position and momentum of an object can in principle be simultaneously determined to any degree of accuracy. If the position and velocity were known precisely at any given moment, then the path of an object, past, present and future, could also be exactly mapped out. These long-established concepts of everyday physics 'can also be defined exactly for the atomic processes', said Heisenberg.41 However, the limitations of these concepts are laid bare when attempts are made to measure simultaneously a pair of conjugate variables: position and momentum or energy and time.
For Heisenberg the uncertainty principle was the bridge between the observation of what appeared to be electron tracks in a cloud chamber and quantum mechanics. As he built that bridge between theory and experiment, he assumed that 'only such experimental situations can arise in nature as can be expressed in the mathematical formalism' of quantum mechanics.42 He was convinced that if quantum mechanics said it could not happen, then it did not. 'The physical interpretation of quantum mechanics is still full of internal discrepancies,' Heisenberg wrote in his uncertainty paper, 'which show themselves in arguments about continuity versus discontinuity and particle versus wave.'43
It was a sorry state of affairs that arose because concepts that had been the foundation of classical physics ever since Newton 'fit nature only inaccurately' at the atomic level.44 He believed that with a more precise analysis of concepts such as position, momentum, velocity, and the path of an elec-tron or atom it might be possible to eliminate 'the contradictions evident up to now in the physical interpretations of quantum mechanics'.45
What is meant by 'position' in the quantum realm? Nothing more or less, Heisenberg answered, than the result of a specific experiment designed to measure, say, the 'position of the electron' in space at a given moment, 'otherwise this word has no meaning'.46 For him there simply is no electron with a well-defined position or a well-defined momentum in the absence of an experiment to measure its position or momentum. A measurement of an electron's position creates an electron-with-a-position, while a measurement of its momentum creates an electron-with-a-momentum. The very idea of an electron with a definite 'position' or 'momentum' is meaningless prior to an experiment that measures it. Heisenberg had adopted an approach to defining concepts through their measurement that harked back to Ernst Mach and what philosophers called operationalism. But it was more than just a redefinition of old concepts.
With the track left behind by an electron passing through a cloud chamber firmly on his mind, Heisenberg examined the concept of the 'path of the electron'. A path is an unbroken, continuous series of positions taken up by the moving electron in space and time. Under his new criteria, to observe the path involves measuring the electron's position at each successive point. However, hitting the electron with a gamma ray photon in the act of measuring its position disturbs it, therefore its future trajectory cannot be predicted with certainty. In the case of an atomic electron 'orbiting' a nucleus, a gamma ray photon is energetic enough to knock it out of the atom, and only one point in its 'orbit' is measured and therefore known. Since the uncertainty principle forbids an exact measurement of both the position and velocity that define the path of an electron or its orbit in an atom, there simply is no path or orbit. The only thing that is known for certain, says Heisenberg, is one point along the path, and 'therefore here the word "path" has no definable meaning'.47 It is measurement that defines what is being measured.
There is no way of knowing, argued Heisenberg, what happens between two consecutive measurements: 'It is of course tempting to say that the electron must have been somewhere between the two observations and that therefore the electron must have described some kind of path or orbit even if it may be impossible to know which path.'48 Tempting or not, he maintained that the classical notion of an electron's trajectory being a continuous, unbroken path through space is unjustified. An electron track observed in a cloud chamber only 'looks' like a path, but is really nothing more than a series of water droplets left in its wake.
Heisenberg was desperately trying to understand the sort of questions that it was possible to answer experimentally after his discovery of the uncertainty principle. It was an unspoken basic tenet of classical physics that a moving object possessed both a precise location in space at a given time and a precise momentum, irrespective of whether it was measured or not. From the fact that the position and momentum of an electron cannot be measured with absolute accuracy at the same time, Heisenberg asserted that the electron does not possesses precise values of 'position' and 'momentum' simultaneously. To talk as if it did, or that it has a 'trajectory', is meaningless. To speculate about the nature of reality that lies beyond the realm of observation and measurement is pointless.
![]()
In later years, Heisenberg repeatedly chose to highlight the moment he remembered his talk with Einstein in Berlin as the crucial juncture on his journey to the uncertainty principle. Yet as he travelled the road to discovery that ended in the depths of a winter's night in Copenhagen, others had walked parts of the route with him. His most influential and valued companion was not Bohr, but Wolfgang Pauli.
As Schrödinger, Bohr and Heisenberg were locked in debate in Copenhagen in October 1926, Pauli was in Hamburg quietly analysing the collision of two electrons. He discovered, aided by Born's probabilistic interpretation, what he described in a letter to Heisenberg as a 'dark point'. Pauli had found that when electrons collide their respective momenta 'must be taken as controlled' and their positions 'uncontrolled'.49 A probable change in momentum was accompanied by a simultaneous but indeterminable change in position. He had found that one could not 'ask simultaneously' about momentum (q) and position (p).50 'One can see the world with the p-eye and one can view it with the q-eye,' Pauli stressed, 'but if one opens both eyes together, then one goes astray.'51 Pauli took it no further, but his 'dark point' lurked in the back of Heisenberg's mind as he and Bohr grappled with the problem of interpretation and wave-particle duality in the months before the discovery of the uncertainty principle.
On 23 February 1927, Heisenberg wrote a fourteen-page letter to Pauli summarising his work on the uncertainty principle. He relied on the critical judgement of the Viennese 'Wrath of God' more than most. 'Day is dawning in quantum theory', replied Pauli.52 Any lingering doubts vanished and, by 9 March, Heisenberg had turned the contents of his letter into a paper for publication. It was only then that he wrote to Bohr in Norway: 'I believe that I have succeeded in treating the case where both [the momentum] p and [the position] q are given to a certain accuracy … I have written a draft of a paper about these problems which yesterday I sent Pauli.'53
Heisenberg chose not to send Bohr either a copy of the paper or the details of what he had done. It was a sign of how strained their relationship had become. 'I wanted to get Pauli's reactions before Bohr was back because I felt again that when Bohr comes back he will be angry about my interpretation', he explained later.54 'So I first wanted to have some support, and see whether somebody else liked it.' Five days after Heisenberg posted his letter, Bohr was back in Copenhagen.
Refreshed after his month-long vacation, Bohr dealt with pressing institute business before carefully reading the uncertainty paper. When they met to discuss it, he told a stunned Heisenberg that it was 'not quite right'.55 Bohr not only disagreed with Heisenberg's interpretation, but he had also spotted an error in the analysis of the gamma-ray microscope thought experiment. The workings of the microscope had nearly proved to be Heisenberg's undoing as a student in Munich. Only the intervention of Sommerfeld had secured his doctorate. Afterwards, a contrite Heisenberg had read up on microscopes, but he was about to discover that he still had some more to learn.
Bohr told Heisenberg it was wrong to place the origin of the uncertainty in the momentum of the electron in the discontinuous recoil it suffers due to the collision with the gamma-ray photon. What prohibits the precise measurement of the momentum of the electron is not the discontinuous and uncontrollable nature of the momentum change, Bohr argued, but the impossibility of measuring that change exactly. The Compton effect, he explained, allows the change in momentum to be calculated with pinpoint accuracy as long as the angle by which the photon is scattered after the collision through the aperture of the microscope is known. However, it is impossible to fix the point where the photon enters the microscope. Bohr identified this as the source of the uncertainty in the momentum of the electron. The electron's position when it collides with the photon is uncertain, since the finite aperture of any microscope limits its resolving power and therefore its ability to locate any microphysical object exactly. Heisenberg had failed to take all this into account, and there was worse to come.
Bohr maintained that a wave interpretation of the scattered light-quantum was indispensable for the correct analysis of the thought experiment. It was the wave-particle duality of radiation and matter that was at the heart of quantum uncertainty for Bohr as he linked Schrödinger's wave packets with Heisenberg's new principle. If the electron is viewed as a wave packet, then for it to have a precise, well-defined position requires it to be localised and not spread out. Such a wave packet is formed from the superposition of a group of waves. The more tightly localised or confined the wave packet is, the greater the variety of waves needed, the greater the range of frequencies and wavelengths involved. A single wave has a precise momentum, but it was an established fact that a group of superimposed waves of differing wavelengths cannot have a well-defined momentum. Equally, the more precisely defined the momentum of a wave packet, the fewer component waves it has and the more spread out it is, thereby increasing the uncertainty in its position. The simultaneously precise measurement of position and momentum is impossible, as Bohr showed that the uncertainty relations could be derived from the wave model of the electron.
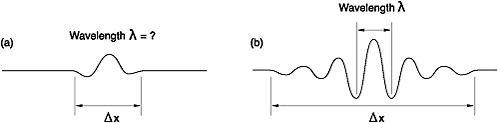
Figure 12: (a) Position of the wave can be precisely determined but not the wavelength (and hence momentum); (b) wavelength can be measured accurately but not the position, since the wave is spread out
What troubled Bohr was that Heisenberg had adopted an approach based exclusively on particles and discontinuity. The wave interpretation, Bohr believed, could not be ignored. He regarded Heisenberg's failure to accommodate wave-particle duality as a deep conceptual flaw. 'I did not know exactly what to say to Bohr's argument,' Heisenberg said later, 'so the discussion ended with the general impression that now Bohr has again shown that my interpretation is not correct.'56 He was furious and Bohr upset at the reaction of his young protégé.
Living next to door to each other and with their offices on the ground floor of the institute separated only by a staircase, Bohr and Heisenberg did well to avoid one another for a few days before meeting again to discuss the uncertainty paper. Bohr hoped that, having had time to cool down, Heisenberg would see reason and rewrite it. He refused. 'Bohr tried to explain that it was not right and I shouldn't publish the paper', Heisenberg said later.57 'I remember that it ended by my breaking out in tears because I just couldn't stand this pressure from Bohr.'58 There was too much at stake for him to simply make the changes being demanded.
Heisenberg's reputation as the wunderkind of physics rested on his discovery of matrix mechanics aged just 24. The growing popularity of Schrödinger's wave mechanics threatened to overshadow, even undermine, that astonishing achievement. Before long he was complaining about the number of papers being written that simply reworked into the language of wave mechanics results first obtained using matrix methods. Although he too had employed the alternative to matrix mechanics as a handy set of mathematical tools with which to calculate the spectrum of helium, Heisenberg harboured hopes of slamming the door on Schrödinger's wave mechanics and the Austrian's claims at having restored continuity. With the discovery of the uncertainty principle, and his interpretation of it based on particles and discontinuity, Heisenberg thought he had closed the door and locked it. He wept tears of frustration as he tried to prevent Bohr from opening it again.
Heisenberg believed that his future was intimately bound to whether it was particles or waves, discontinuity or continuity that ruled in the atomic domain. He wanted to publish as quickly as possible and challenge Schrödinger's claim that matrix mechanics was unanschaulich, unvisualisable, and therefore untenable. Schrödinger disliked discontinuity and a particle-based physics as much as Heisenberg loathed a physics of continuity and waves. Armed with the uncertainty principle and what he deemed to be the correct interpretation of quantum mechanics, Heisenberg went on the attack as he consigned his rival to a footnote in his paper: 'Schrödinger describes quantum mechanics as a formal theory of frightening, indeed repulsive, abstractness and lack of visualizability. Certainly one cannot overestimate the value of the mathematical (and to that extent physical) mastery of the quantum-mechanical laws that Schrödinger's theory has made possible. However, as regards questions of physical interpretation and principle, the popular view of wave mechanics, as I see it, has actually deflected us from exactly those roads which were pointed out by the papers of Einstein and de Broglie on the one hand and by the papers of Bohr and by quantum mechanics [i.e. matrix mechanics] on the other hand.'59
On 22 March 1927, Heisenberg posted his paper, 'On the perceptual content of quantum theoretical kinematics and mechanics', to the Zeitschrift für Physik, the quantum theorist's journal of choice.60 'I quarrel with Bohr', he wrote to Pauli two weeks later.61 'By exaggerating one side or the other,' protested Heisenberg, 'one can discuss a lot without saying anything new.' Believing that he had dealt with Schrödinger and his wave mechanics once and for all, Heisenberg now faced a far more tenacious opponent.
While Heisenberg was busy exploring the consequences of the uncertainty principle in Copenhagen, on the ski slopes in Norway, Bohr came up with complementarity. It was for him no mere theory or a principle, but the necessary conceptual framework hitherto missing for describing the strange nature of the quantum world. Complementarity, Bohr believed, could accommodate the paradoxical nature of wave-particle duality. The wave and particle properties of electrons and photons, matter and radiation, were mutually exclusive yet complementary aspects of the same phenomenon. Waves and particles were two sides of the same coin.
Complementarity neatly sidestepped the difficulties that arose from having to use two disparate classical descriptions, waves and particles, to describe a non-classical world. Both particles and waves were, according to Bohr, indispensable for a complete description of quantum reality. Either description by itself is only partially true. Photons paint one picture of light, waves another. Both hang side by side. But to avoid contradictions, there were limitations. The observer can look at only one of them at any given time. No experiment would ever reveal a particle and a wave at the same time. Bohr argued that 'evidence obtained under different conditions cannot be comprehended within a single picture, but must be regarded as complementary in the sense that only the totality of the phenomena exhausts the possible information about the objects'.62
Bohr found support for his emerging ideas when he saw something in the uncertainty relations, ![]() p
p![]() q
q![]() h/2
h/2![]() and
and ![]() E
E![]() t
t![]() h/2
h/2![]() , that Heisenberg, blinded by his intense dislike of waves and continuity, did not. The Planck-Einstein equation E=hv and de Broglie's formula p=h/
, that Heisenberg, blinded by his intense dislike of waves and continuity, did not. The Planck-Einstein equation E=hv and de Broglie's formula p=h/![]() embodied wave-particle duality. Energy and momentum are properties commonly associated with particles, whereas frequency and wavelength are both characteristics of waves. Each equation contained one particle-like and one wave-like variable. The meaning of this combination of particle and wave characteristics in the same equation was something that niggled Bohr. After all, a particle and a wave are two wholly distinct physical entities.
embodied wave-particle duality. Energy and momentum are properties commonly associated with particles, whereas frequency and wavelength are both characteristics of waves. Each equation contained one particle-like and one wave-like variable. The meaning of this combination of particle and wave characteristics in the same equation was something that niggled Bohr. After all, a particle and a wave are two wholly distinct physical entities.
As he corrected Heisenberg's analysis of the microscope thought experiment, Bohr spotted that the same was true for the uncertainty relations. It was a finding that led him to interpret the uncertainty principle as revealing the extent to which two complementary but mutually exclusive classical concepts, either particles and waves or momentum and position, could be applied simultaneously without contradiction in the quantum world.63
The uncertainty relations also implied that a choice has to be made between what Bohr called a 'causal' description based on the conservation laws of energy and momentum (E and p in the uncertainty relations), and a 'space-time' description in which events are followed in space and time (q and t). The two descriptions were mutually exclusive but complementary so as to account for the results of all possible experiments. To Heisenberg's dismay, Bohr had reduced the uncertainty principle to a special rule exposing the limits inherent in nature on any simultaneous measurements of complementary pairs of observables such as position and momentum or on the simultaneous use of two complementary descriptions.
There was another difference of opinion. Whereas the uncertainty principle led Heisenberg to question the extent to which classical concepts such as 'particle', 'wave', 'position', 'momentum' and 'trajectory' were applicable in the atomic realm, Bohr argued that the 'interpretation of the experimental material rests essentially upon the classical concepts'.64 While Heisenberg insisted upon an operational definition of these concepts, a sort of meaning through measurement, Bohr argued that their meanings were already fixed by how they were used in classical physics. 'Every description of natural processes,' he had written in 1923, 'must be based on ideas which have been introduced and defined by the classical theory.'65 Regardless of any limitations imposed by the uncertainty principle, they could not be replaced for the simple reason that all experimental data, its discussion and interpretation, by which theories are put to the test in the laboratory, is of necessity expressed in the language and concepts of classical physics.
Heisenberg suggested that since classical physics was found wanting at the atomic level, why should these concepts be retained? 'Why should we not simply say that we cannot use these concepts with a very high precision, therefore the uncertainty relations, and therefore we have to abandon these concepts to a certain extent', he argued in the spring of 1927.66 When it comes to the quantum, 'we must realize that our words don't fit'. If words fail, then the only sensible option for Heisenberg was to retreat into the formalism of quantum mechanics. After all, he maintained, 'a new mathematical scheme is just as good as anything because the new mathematical scheme then tells what may be there and what may not be there'.67
Bohr was unconvinced. The gathering of every piece of information about the quantum world, he pointed out, involves performing an experiment the results of which are recorded as fleeting flashes of light on a screen, or as clicks of a Geiger counter, or registered by the movement of needles on voltmeters and the like. Such instruments belong to the everyday world of the physics laboratory, but they are the only means by which an event at the quantum level can be magnified, measured, and recorded. It is the interaction between a piece of laboratory equipment and a microphysical object, an alpha particle or an electron, which triggers the click of a Geiger counter or causes the needle of a voltmeter to move.
Any such interaction involves the exchange of at least one quantum of energy. The consequence of this, Bohr said, is the 'impossibility of any sharp distinction between the behaviour of atomic objects and the interactions with the measuring instruments which serve to define the conditions under which the phenomena appear'.68 In other words, it was no longer possible to make the separation that existed in classical physics between the observer and the observed, between the equipment used to make a measurement and what was being measured.
Bohr was adamant that it was the specific experiment being performed that revealed either the particle or wave aspects of an electron or a beam of light, of matter or radiation. Since particle and wave were complementary but mutually exclusive facets of one underlying phenomenon, in no actual or imaginary experiment could both be revealed. When equipment was set up to investigate the interference of light, as in Young's famous two-slits experiment, it was the wave nature of light that was manifest. If it was an experiment to study the photoelectric effect by shining a beam of light onto a metal surface, then it was light as a particle that would be observed. To ask whether light is either a wave or a particle is meaningless. In quantum mechanics, said Bohr, there is no way of knowing what light 'really is'. The only question worth asking is: Does the light 'behave' like a particle or a wave? The answer is that sometimes it behaves like a particle and at others like a wave, depending upon the choice of experiment.
Bohr assigned a pivotal role to the act of choosing which experiment to perform. Heisenberg identified the act of measurement to determine, for example, the exact position of an electron as the origin of a disturbance that ruled out a simultaneously precise measurement of its momentum. Bohr agreed that there was a physical disturbance. 'Indeed, our usual [classical] description of physical phenomena is based entirely on the idea that the phenomena concerned may be observed without disturbing them appreciably', he said during a lecture delivered in September 1927.69 It was a statement implying that such a disturbance is caused by the act of observing phenomena in the quantum world. A month later he was more explicit when, in a draft of a paper, he wrote 'that no observation of atomic phenomena is possible without their essential disturbance'.70 However, he believed that the origin of this irreducible and uncontrollable disturbance lay not in the act of measurement but in the experimenter having to choose one side of the wave-particle duality in order to perform that measurement. Uncertainty, Bohr argued, was nature's price for making that choice.
In the middle of April 1927, as he worked on formulating a consistent interpretation of quantum mechanics within the conceptual framework provided by complementarity, Bohr sent a copy of the uncertainty paper to Einstein at Heisenberg's request. In the accompanying letter he wrote that it was a 'very important contribution to the discussion of the general problems of quantum theory'.71 In spite of their ongoing and often heated arguments, Bohr informed Einstein that 'Heisenberg shows in an exceedingly brilliant manner how his uncertainty relations may be utilized not only in the actual development of quantum theory, but also for the judgement of its visualizable content'.72 He went on to outline some of his own emerging ideas that would throw light on 'the difficulties of the quantum theory [that] are connected with the concepts, or rather with the words that are used in the customary description of nature, and which always have their origin in the classical theories'.73 Einstein, for some unknown reason, chose not to reply.
If he was hoping to elicit a response from Einstein, then Heisenberg must have been disappointed when he returned to Copenhagen after spending Easter in Munich. It was a much-needed break from the constant pressure to yield to Bohr's interpretation. 'So I have come to be in a fight for the matrices and against the waves', Heisenberg wrote to Pauli on 31 May, the very day his 27-page paper appeared in print. 'In the ardour of this struggle I have often criticized Bohr's objections to my work too sharply and, without realizing or intending it, have in this way personally wounded him. When I now reflect on these discussions, I can very well understand that Bohr was angry about them.'74 The reason for such contrition was that two weeks earlier, he had finally admitted to Pauli that Bohr was right.
The scattering of gamma rays into the aperture of the hypothetical microscope was the basis of the uncertainty relation for momentum and position. 'Thus the relation ![]() p
p![]() q
q![]() h indeed comes out naturally, but not entirely as I had thought.'75 Heisenberg went on to concede that 'certain points' were easier to handle using Schrödinger's wave description, but he remained utterly convinced that in quantum physics 'only discontinuities are interesting' and they could never be emphasised enough. It was still not too late to withdraw the paper, but it was a step too far. 'All results of the paper are correct after all,' he told Pauli, 'and I am also in agreement with Bohr concerning these.'76
h indeed comes out naturally, but not entirely as I had thought.'75 Heisenberg went on to concede that 'certain points' were easier to handle using Schrödinger's wave description, but he remained utterly convinced that in quantum physics 'only discontinuities are interesting' and they could never be emphasised enough. It was still not too late to withdraw the paper, but it was a step too far. 'All results of the paper are correct after all,' he told Pauli, 'and I am also in agreement with Bohr concerning these.'76
As a compromise, Heisenberg added a postscript. 'After the conclusion of the foregoing paper,' it began, 'more recent investigations of Bohr have led to a point of view which permits an essential deepening and sharpening of the analysis of quantum-mechanical correlations attempted in this work.'77 Heisenberg acknowledged that Bohr had brought to his attention crucial points that he had overlooked - uncertainty was a consequence of wave-particle duality. He closed by thanking Bohr, and with the publication of the paper, months of wrangling and 'gross personal misunderstandings', though not entirely forgotten, were firmly pushed aside.78 Whatever their differences, as Heisenberg said later, 'all that mattered now was to present the facts in such a way that despite their novelty they could be grasped and accepted by all physicists'.79
'I am very ashamed to have given the impression of being quite ungrateful', Heisenberg wrote to Bohr in the middle of June, not long after Pauli had visited Copenhagen.80 Two months later, still full of remorse, he explained to Bohr how he reflected 'almost every day on how that came about and am ashamed that it could not have gone otherwise'.81 Future job prospects had been a major determining factor in the rush to publish. When he turned down the Leipzig professorship in favour of Copenhagen, Heisenberg was certain that if he continued producing 'good papers', then universities would come calling.82 After the publication of the uncertainty paper, the job offers came. Anxious that Bohr might think otherwise, he was quick to explain that he had not encouraged potential suitors because of their recent dispute over uncertainty. Not yet 26, Heisenberg became Germany's youngest ordinary professor when he accepted a new offer from Leipzig University. He left Copenhagen at the end of June. By then life at the institute was back to normal, as Bohr continued the painfully slow business of dictating the paper on complementarity and its implications for the interpretation of quantum mechanics.
He had been hard at work on it since April, and Oskar Klein, a 32-year-old Swede based at the institute, was the person Bohr turned to for help. As the argument over uncertainty and complementarity raged, Hendrik Kramers, Bohr's former assistant, warned Klein: 'Do not enter this conflict, we are both too kind and gentle to participate in that kind of struggle.'83 When Heisenberg first learnt that Bohr was writing a paper aided by Klein on the basis that 'there exists waves and particles', he wrote rather disparagingly to Pauli that 'when one starts like that, then one can of course make everything consistent'.84
As one draft followed another and the title changed from 'The philosophical foundations of the quantum theory' to 'The quantum postulate and the recent development of atomic theory', Bohr tried hard to finish the paper so he could present it at a forthcoming conference. But it turned out to be yet another draft. For the time being, it would have to do.
![]()
The International Physics Congress from 11 to 20 September 1927 in Como, Italy was held to commemorate the 100th anniversary of the death of the Italian Alessandro Volta, the inventor of the battery. With the conference in full swing, Bohr was still finalising his notes until the day of the lecture on 16 September. Among the audience at the Istituto Carducci eager to hear what he had to say were Born, de Broglie, Compton, Heisenberg, Lorentz, Pauli, Planck, and Sommerfeld.
It was impossible for some in the audience to catch every softly spoken word that followed as Bohr outlined for the first time his new framework of complementarity, followed by an exposition of Heisenberg's uncertainty principle and the role of measurement in quantum theory. Bohr stitched each of these elements together, including Born's probabilistic interpretation of Schrödinger's wave function, so that they constituted the foundations of a new physical understanding of quantum mechanics. Physicists would later call this fusion of ideas the 'Copenhagen interpretation'.
Bohr's lecture was the culmination of what Heisenberg later described as 'an intensive study of all questions concerning the interpretation of quantum theory in Copenhagen'.85 At first even the young quantum magician was uneasy with the Dane's answers. 'I remember discussions with Bohr which went through many hours till very late at night and ended almost in despair,' Heisenberg wrote later, 'and when at the end of the discussion I went alone for a walk in the neighbouring park I repeated to myself again and again the question: Can nature possibly be as absurd as it seemed to us in these atomic experiments?'86 Bohr's answer was an unequivocal yes. The central role given to measurement and observation vitiated all attempts to unearth regular patterns in nature or any causal connections.
It was Heisenberg, in his uncertainty paper, who first advocated in print the rejection of one of the central tenets of science: 'But what is wrong in the sharp formulation of the law of causality, "When we know the present precisely, we can predict the future," is not the conclusion but the assumption. Even in principle we cannot know the present in all detail.'87 Not knowing simultaneously the exact initial position and velocity of an electron, for example, allows only probabilities of a 'plenitude of possibilities' of future positions and velocities to be calculated.88 Therefore it is impossible to predict the exact result of any single observation or measurement of an atomic process. Only the probability of a given outcome among a range of possibilities can be precisely predicted.
The classical universe built on the foundations laid down by Newton was a deterministic, clockwork cosmos. Even after Einstein's relativistic remodelling, if the exact position and velocity of an object, particle or planet, are known at any given moment, then in principle its position and velocity can be completely determined for all time. In the quantum universe there was no room for the determinism of the classical, where all phenomena can be described as a causal unfolding of events in space and time. 'Because all experiments are subject to the laws of quantum mechanics, and therefore to equation ![]() p
p![]() q
q![]() h,' Heisenberg boldly asserted in the last paragraph of his uncertainty paper, 'it follows that quantum mechanics establishes the final failure of causality.'89 Any hope of restoring it was as 'fruitless and senseless' as any lingering belief in a 'real' world hidden behind what Heisenberg called 'the perceived statistical world'.90 It was a view shared by Bohr, Pauli and Born.
h,' Heisenberg boldly asserted in the last paragraph of his uncertainty paper, 'it follows that quantum mechanics establishes the final failure of causality.'89 Any hope of restoring it was as 'fruitless and senseless' as any lingering belief in a 'real' world hidden behind what Heisenberg called 'the perceived statistical world'.90 It was a view shared by Bohr, Pauli and Born.
At Como two physicists were noticeable by their absence. Schrödinger had only weeks earlier moved to Berlin as Planck's successor and was busy settling in. Einstein refused to set foot in fascist Italy. Bohr would have to wait just a month before they met in Brussels.