ESCAPING FROM PREDATORS An Integrative View of Escape Decisions (2015)
Part II Escape and refuge use: theory and findings for major taxonomic groups
IIb Escape decisions prior to pursuit
7Invertebrates
Philip W. Bateman and Patricia A. Fleming
7.1 Introduction
Invertebrates comprise an estimated 80% of all multicellular animals. Understanding their antipredator responses therefore makes a substantial contribution to our knowledge of animal responses to predators. The diversity of invertebrates, including a range of lifestyles, body forms, and habitat use, means that day to day, they encounter a similarly wide range of risky situations. Surprisingly, however, there are actually few studies that have examined invertebrate escape behavior from the point of view of the Economic Escape Model (EEM). Of the studies carried out, there has been a bias toward decapods (e.g., crabs), with notable contributions from Lepidoptera (particularly moths), Orthoptera (crickets and grasshoppers), and Arachnida (spiders).
It is possible that the paucity of data for invertebrate escape behavior may be due to methodological challenges. Firstly, multicellular invertebrates are generally smaller than vertebrates. Invertebrates might therefore face a wider range of predator sizes than vertebrates, including a higher likelihood of “accidental” predation through events such as being ingested with foliage by herbivores (Ben-Ari & Inbar 2013). Because they are so small in size, we need to be cautious to not overinterpret the responses of invertebrates. While, to human observers, it may be intuitive how vertebrates react to approaching predators or disturbance (e.g., “alert” behavior, cessation of previous behavior, gaze direction, alarm calling), this is less clear for many invertebrates. Secondly, invertebrates perceive their world through a wide range of sensory modalities: in addition to sight and sound, they also respond to chemicals, air pressure, vibration or changes in light level. Locusts, for example, detect potential threats through a wide range of senses (Table 7.1). These different modes of sensitivity to their environment often mean that we have to think “outside the box” when working with invertebrates, to ensure that we correctly assess the stimuli and consequent responses.
Table 7.1 Avoidance behavior of locusts in response to stimuli has been described for several sensory modalities.
|
Receptors |
Responses |
|
Mechanoreceptors |
Tactile hair receptors on different regions of the body can elicit specific avoidance responses ranging from simple retraction of a leg to active defense. |
|
e.g., wind receptors: setae on the dorsal surface of the head, which when stimulated with air currents, cause wing flapping for as long as the wind flows (Camhi 1969). |
|
|
Auditory input from ultrasound sources induces avoidance steering. |
|
|
Chemoreceptors |
Chemical cues from leg contact chemoreceptors elicit avoidance by setting the tarsus into a new position. |
|
Thermoreceptors |
Heat (infrared radiation) is avoided by flying locusts. |
|
Photoreceptors |
Visual cues reliably initiate the aversive reactions of collision avoidance in flying locusts. Expanding shapes elicit steering responses that have been interpreted as collision-avoidance strategies in different flight situations. Several large visual interneurones descending from the brain react to expanding shapes and may therefore contribute to these visually elicited avoidance responses. |
(Modified from Hassenstein & Hustert, 1999, and references therein)
Identifying common responses to being approached by a predator can be a challenge in interpreting invertebrate antipredator behavior. However, this is helped by recognizing that their escape behavior can be effectively pared down to the simplest responses to stimuli. A recent review (Card 2012) emphasizes that there are relatively few synapses involved in insect escape behavior, reducing processing time and consequently resulting in remarkably rapid responses. However, Card (2012) also points out that even the escape of a fly is a series of “sub-behaviors” that allow adaptive abortion of the process, i.e., it is not a fixed action pattern and is therefore potentially highly flexible.
In addition to their diversity and abundance, invertebrates can also carry evidence of past predatory encounters, which may modify their responses. Many invertebrates undergo autotomy - the voluntary shedding of a limb or other part of the body along a breakage plane (reviewed by Fleming et al. 2007). Autotomy happens as a result of entrapment (usually when grabbed by a potential predator), and therefore an autotomized body part directly reflects a successful previous escape. Most invertebrates that have lost a leg have reduced locomotory ability (reviewed by Fleming et al. 2007). Animals may also autotomize an appendage that could have been used in self-defense, e.g., the chelipeds of crabs (Juanes & Smith 1995). Autotomy can therefore render an individual more vulnerable to future encounters with a predator (McPeek et al. 1996; Stoks 1998, 1999; Gyssels & Stoks 2005; Bateman & Fleming 2006a) and will potentially influence economic escape decisions (Cooper & Frederick 2010).
7.2 Measures of invertebrate escape response
Invertebrates use a diversity of antipredator behavior, including cessation of movement, falling silent, dropping, taking flight (walking, running, or flying), or retreating to cover, as appropriate to their environment, morphology, and ecology (Figure 7.1). In this section, we discuss these antipredator responses in terms of the measures that can be made.
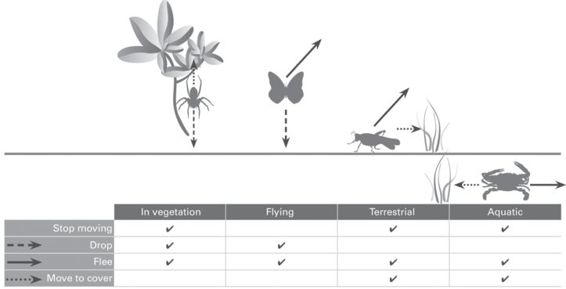
Figure 7.1
A range of behavioral options are available for invertebrates that usually are found within vegetation, fly, or are epigeic. Crypsis is an important primary defense (functioning regardless of whether a predator is present or not; sensu Edmunds 1974), but when crypsis fails, invertebrates must resort to a range of secondary defense responses.
7.2.1 Watching and waiting
Measuring predator monitoring in invertebrates can be problematic because, to a human observer, it is not always evident when an invertebrate is “alert” to the predator’s presence or has modified its behavior accordingly (i.e., is “monitoring” its environment). Below we discuss where monitoring is evident because animals stop their previous actions, alter their body position, or retreat to cover.
Fiddler crabs Uca vomeris visually monitor approaching predatory stimuli until deciding to retreat to their burrows (Hemmi 2005a, b), but also continue to monitor them from the entrance of their burrows before retreating wholly (Hemmi & Pfeil 2010). Uca pugilator fiddler crabs retreat to burrows if they see conspecifics reacting to threats, even if they do not see the threat themselves (Wong et al. 2005). Uca pugilator are therefore monitoring their conspecifics for cues about potential predators.
7.2.1.1 Cessation of movement
Cessation of movement, tonic immobility, or thanatosis (where the organism effectively plays dead) is a first line of defense for several invertebrate species. Stopping moving is typical of cryptic species (e.g., Hatle & Faragher 1998). Xanthodius sternberghii crabs disturbed by human activity initially rely on keeping still (which is effective due to their cryptic coloration), but, after a minute, the crabs will either slowly edge for cover or imperceptibly dig themselves into the sand (Robinson et al. 1970).
Larvae of the damselfly Ischnura elegans that have autotomized lamellae (used for gas exchange and locomotion) through unsuccessful predation events enter thanatosis more frequently when exposed to a predatory stimulus than intact individuals (Gyssels & Stoks 2005). In ponds where these damselfly larvae have only invertebrate predators, this form of defense may be sufficient to avoid predation. However, predatory fish are able to locate larvae whether they are immobile of not, and larvae from ponds with fish predators are less likely to become immobile and stay immobile for shorter lengths of time than are larvae from ponds without fish predators.
Ohno and Miyatake (2007) demonstrated a trade-off (a negative genetic correlation) between different responses (thanatosis and flying ability) to potential predation in bean beetles Callosobruchus chinensis. This suggests that, within a population, there may be groups of individuals that are more or less likely to resort to one of these two mutually exclusive antipredator tactics. The propensity of ceasing movement also appears to be influenced by what an individual was doing before being disturbed. Sweet potato beetles Cylas formicarius that were moving when disturbed are less likely to enter thanatosis compared with individuals that were already still (Miyatake 2001).
Cessation of movement probably comes with minimal energetic costs, but since we know very little about the mechanisms that cause tonic immobility in invertebrates, it is difficult to assess whether there are any indirect effects of monitoring risk until they recommence movement. The biggest costs to such behavior are likely to be opportunity costs and cost of monitoring the environment.
7.2.1.2 Falling silent
For organisms that signal acoustically, falling silent is another way of enhancing crypsis when a potential predator is detected. Orthopterans that are acoustically signaling cease movement and fall silent when disturbed by a predatory stimulus (Hedrick 2000; Bateman & Fleming 2013b). Male armored ground crickets, Acanthoplus speiseri, stridulate both during the day and night. They fall silent and initially rely on crypsis when approached by a human. These crickets use visual cues to recognize the intruder during the day, stopping calling when the person is still some distance away, but at night they can be approached and touched before they fall silent (Bateman & Fleming 2013b).
Animals may then remain silent for varying lengths of time; this latency until the resumption of signaling may be influenced by risk assessment. Their latency to resume signaling is influenced by perceived presence of predators (Zuk & Kolluru 1998; Lewkiewicz & Zuk 2004) but it can also vary according to previous experience with predators: crickets that autotomized a limb in a predatory encounter and thus have reduced locomotory ability (Bateman & Fleming 2005, 2006a) increase and maintain a high latency to begin calling again postdisturbance (Bateman & Fleming 2006b).
7.2.1.3 “Positioning” behavior
Between cessation of moving and active flight away from a predator is a small suite of behavioral actions that we term “positioning behavior.” Many grasshoppers perched on vegetation reposition themselves on the other side of a stem or leaf from the approaching predator (“squirreling”). Other positioning/hiding responses include “jerking” (short, lateral movements), “crouching,” and small backward motions (Hassenstein & Hustert 1999). Locusts Locusta migratoria squirrel around stem perches in response to visual stimuli, i.e., dark, moving, or expanding shapes. Interestingly, they do not respond to acoustic stimuli, although noises prior to a visual stimulus may increase their propensity to hide (Hassenstein & Hustert 1999).
The likelihood of squirreling may be influenced by body condition. Semi-aquatic grasshoppers Paroxya atlantica that had autotomized a hind limb show a greater likelihood to squirrel from an approaching observer than intact individuals (Bateman & Fleming 2011). They also perch lower on emergent vegetation than intact individuals, presumably in readiness for escape via water.
This sort of behavior is likely to be energetically inexpensive, is less likely to attract a predator’s attention than fleeing, has a lower latency until normal behavior can be resumed, and allows continued monitoring of the predator.
7.2.2 Fleeing
If keeping still and/or silent is not sufficient to avoid detection by a predator, prey have the option to flee. The various forms of fleeing reflect the wide range of prey biology.
7.2.2.1 Dropping
The simplest form of “fleeing,” which is common to many arthropods on vegetation or in flight, is to drop vertically toward the ground (aided by gravity), out of the way of the predator or an approaching disturbance. Colonial spiders Metepeira incrassata drop out of communal nests into vegetation below when predatory wasps attack the nest (Uetz et al. 2002). Caterpillars of the lymantrid moth Orgyia leucostigma rely on their coating of bristly hairs, not only as a primary defense of a barrier against attack, but also as sensors (Castellanos et al. 2011). Low hair-bending velocity results in the caterpillar walking away from the disturbance, but high hair-bending velocity results in dropping from their perch, presumably as it is interpreted as a higher risk predatory stimulus.
Dropping is an important antipredator response of flying invertebrates. Various insects have evolved the ability to acoustically detect predatory bats and dramatically drop from the air to avoid them. Green lacewings Chrysopa carnea fold their wings and nose dive in response to the echolocation calls of bats (Miller & Olesen 1979). Many species of moths, crickets, bush crickets, mantids, and some flies and beetles show similar bat-avoidance behavior (Miller & Surlykke 2001). Female parasitic flies Ormia ochracea perform phonotaxis at night to their victims, which are singing male crickets (Rosen et al. 2009). Like female crickets performing phonotaxis to the males, these flies also drop from the air in response to bat echolocation calls.
Arthropods also drop from plants even when the disturbance is unintentional. Aphids drop when herbivores feed on the plants they are on (Gish et al. 2010, 2011). Up to 14% of pea aphids Acyrthosiphon pisum feeding on alfalfa bushes drop to the ground when hemipteran predators are introduced to the bush, and up to 60% of the aphids drop when the highly predatory ladybird Coccinella septempunctata is placed on the bush (Losey & Denno 1998). Pea aphids also drop from food plants when they detect alarm pheromones from conspecifics indicating active predation (Dill et al. 1990). Both larvae and adults of three coccinellid beetle species drop when mammalian herbivores feed on the plants they are on (Ben-Ari & Inbar 2013). When breathed on by humans, 60 to 80% of the beetles drop, probably reacting to heat and humidity, but they do not drop if the plant is merely shaken (Ben-Ari & Inbar 2013).
Dropping comes with costs, such as cessation of feeding, desiccation (for sap-feeding insects), increased locomotory costs in regaining their position, or greater exposure to animals on the ground. Pea aphids therefore “assess” risk and are less likely to drop when the environment is hot and dry, or when they are feeding on high-quality rather than low-quality host plants (Dill et al. 1990). Because dropping is costly, there needs to be good discrimination between reasonably safe situations vs. truly dangerous stimuli. The dogbane tiger moth Cycnia tenera can differentiate between the echolocation calls of bats that are flying nearby looking for prey (“early attack”) and calls of these bats that have detected prey and are moving into pursuit (“late attack”). When first exposed to bat echolocation calls post-adult molt, moths show equal defensive responses (“startle” calls of ultrasonic clicks and dropping) to both types of call, but soon learn to discriminate between them, and subsequently will continue other behavior in the presence of “early attack” echolocation calls (Ratcliffe et al. 2011). Similar differentiation between “early” and “late” echolocation calls has been recorded for the moth Bertholdia trigona (Corcoran et al. 2013).
7.2.2.2 Taking flight: walking, running, flying, or swimming
When an invertebrate flees from an approaching predator there are various measures that can reveal important information about this response. Measures include FID, DF, angle of flight away from the predator, and the type of flight used (i.e., fast or slow; straight or protean).
7.2.2.2.1 Flight initiation distance (FID)
Under predictions of the EEM, FID should increase in the presence of greater risk (Chapter 2). A number of studies apparently support FID as a measure of level of risk in invertebrates (Table 7.2). Cooper (2006) recorded an increase in FID in Dissosteira carolina grasshoppers that were approached faster, more directly, or twice in succession, while Hemmi (2005a) showed that fiddler crabs Uca vomeris approached by a model seabird showed increased FID when they were further from their burrow.
Table 7.2 Studies of FID in response to varying threat levels to test the EEM in invertebrates.
|
Prey organism |
Stimulus |
Treatment |
Was there a difference in FID? |
Reference |
|
Grasshoppers |
||||
|
- Dissosteira carolina |
Person |
Approach speed |
Yes ↑ for faster approach |
[1] |
|
Directness of approach |
Yes ↑ for direct approach |
[1] |
||
|
Repeated approach |
Yes ↑ for 2nd approach |
[1] |
||
|
- Schistocerca alutacea |
Person |
Approached repeatedly |
No |
[2] |
|
- Psinidia fenestralis |
Person |
Approached repeatedly |
No (Marginally ↑ FID on the second approach, but not for subsequent approaches) |
[2] |
|
- Paroxya atlantica |
Person |
Autotomized vs. intact |
No |
[3] |
|
Armored ground crickets Acanthoplus speiseri |
Person |
Calling males, at night and in the day |
Yes ↓ at night |
[4] |
|
Mayfly larvae Baetis tricaudatus |
Predatory fish and stonefly larvae |
High- vs. low-food patches |
[5] |
|
|
- Large larvae |
Yes ↓ (¼ ×) in high-food patchesa |
|||
|
- Small larvae |
No |
|||
|
Water strider Gerris remigis |
Adult water striders approaching juveniles |
Group size of juveniles |
Yes ↑ for larger groups, then ↓ for even larger groups |
[6] |
|
Wolf spiders Hogna carolinensis |
Model lizard |
Mass, size, sex, and speed of spiders |
Yes ↓for slower individuals |
[7]. |
|
Colonial spiders Metepeira incrassata |
Predatory wasp |
Position in nest, age of spiders |
Yes ↑ for adult females in center of nest (furthest from initiation of wasp attack: No for immature spiders) |
[8] |
|
Orb weaver spiders Argiope florida |
Person or shape |
Person “looming” or small fluttering shape “hovering” |
Nob |
[9] |
|
Fiddler crabs Uca vomeris |
Model seabird |
Distance to refuge |
Yes ↓ FID when closer to refuge |
[10] |
|
Angle of approach (directly or tangentially) |
Yes ↓ FID when approached directly |
[11] |
||
|
Caribbean hermit crabs Coenobita clypeatus |
Person or shape |
Silent or noisy approach |
Yes ↓ “FID” (withdrawing into shell) when distracted by noise |
[12] |
a FID through drift in the stream flow.
b Only effect was height of web from the ground, which could reflect differences in visual contrast from the vegetation.
References: [1]: Cooper, 2006; [2]: Bateman & Fleming, 2014; [3]: Bateman & Fleming, 2011; [4]: Bateman & Fleming, 2013b; [5]: Scrimgeour, et al., 1994; [6]: Dill & Ydenberg, 1987; [7]: Nelson & Formanowicz, 2005; [8]: Uetz et al. 2002; [9]: Bateman & Fleming, 2013a; [10]: Hemmi, 2005a; [11]: Hemmi, 2005b; [12]: Chan, et al. 2010.
Other studies, however, have recorded no increase in FID in response to increased risk. Three other grasshopper species show little evidence for an increase in FID when approached repeatedly or for autotomized grasshoppers (other escape tactics are instead evident, including increases in DF, altering angle of escape, or use of crypsis) (Bateman & Fleming 2011, 2014). Autotomy can influence FID as loss of limb may reduce locomotory ability, such that an increased FID would be adaptive, but lower initial fitness after autotomy, encouraging decreased FID (Cooper & Frederick 2010). This relationship needs more study in invertebrates. Orb-weaver spiders Argiope florida do not vary their FID in response to varying types of approach (Bateman & Fleming 2013b). Hemmi (2005b) reported that Uca vomeris actually show decreased FID when approached directly (rather than tangentially), and Caribbean hermit crabs Coenobita clypeatus similarly show decreased hiding initiation distance (i.e., when they withdraw into their shell) when disturbed by noise (Chan et al. 2010).
We need to be cautious not to overinterpret the results of FID experiments in invertebrates, since the physiological limitations of the animals may contribute to responses. Flight initiation distance may simply vary as a consequence of visual acuity (Box 7.1), or depth perception, or speed of cognitive processing (e.g., Dukas 1998; Bateman & Fleming 2013a; Lee et al. 2013), and individuals may therefore show limited plasticity in FID. Consequently, we might not predict variation in FID within individuals and populations, but we might expect variation between populations that vary in predation intensity that might, for example, select for individuals with a lower response threshold.
Box 7.1 Vision in arthropods
The compound eyes of arthropods come in a number of general patterns. Both simple (or single chambered) and compound (or multifaceted) eyes split up incoming light according to its direction and origin. Compound eyes are of two distinct and optically different kinds. In apposition eyes (most diurnal insects), each cluster of receptors has their own lens. In superposition eyes, such as in Lepidoptera, the image at any point on the retina is the product of many lenses.
Flight responses are triggered by a threshold size and/or speed of a “looming” image on the retina (Rind & Simmons 1992; Javůrková et al. 2012). When the image reaches a threshold size (i.e., number of ommatadia neurons firing), then the escape response will be triggered (Figure 7.2). For example, hiding responses (squirreling to the other side of a branch from the approaching object) in locusts Locusta migratoria, stimulated by expanding shapes, occur only after the expanding image has exceeded a threshold visual angle of 8 to 9.5° (Hassenstein & Hustert 1999).
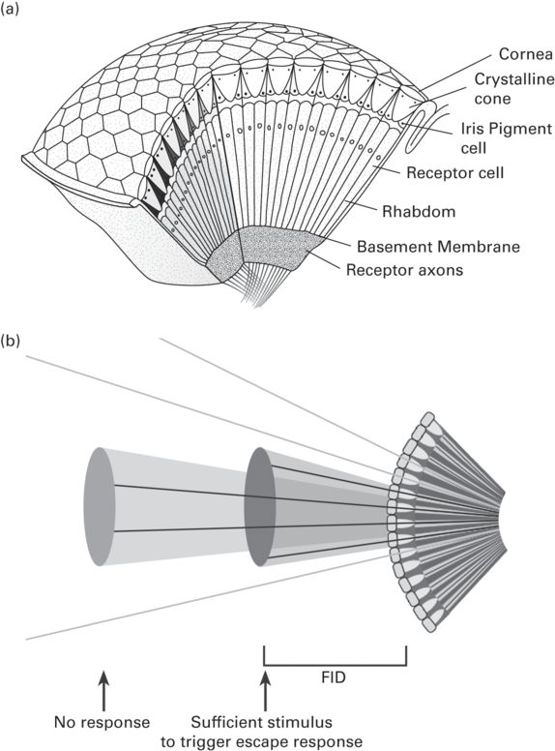
Figure 7.2
(a) Basic structure of an appositon compound eye found in most diurnal insect species, showing its construction from ommatidial elements. (b) A looming shape will cause an escape response when a threshold angle (i.e., number of ommatidia stimulated) are triggered. The distance from the eye when this occurs will therefore determine the FID for the animal.
Fleeing will result in opportunity costs as well as metabolic costs. Interestingly, flight in many invertebrates is particularly efficient, such that the metabolic costs of flight may be fairly minimal. The tracheal system of invertebrates such as a grasshopper is particularly efficient in ensuring oxygen availability to metabolically active tissue, and consequently (unlike vertebrates, which will readily resort to anaerobic respiration during sprint escapes) they are unlikely to have to rely on anaerobic respiration (Weyel & Wegener 1996). Lack of oxygen is rare for such invertebrates (although it can certainly apply to aquatic invertebrates that may encounter hypoxic zones) and, unlike many mammals and birds, they can maintain high metabolic rates and ATP synthesis (Weyel & Wegener 1996). Notably, however, “injury” through autotomy of a limb can increase costs of locomotion and decrease stamina in field crickets (Fleming & Bateman 2007).
7.2.2.2.2 Distance fled (DF)
In a high-risk situation (e.g., when approached by a fast, persistent predator), it would be beneficial to increase DF. Dissosteira carolina grasshoppers approached quickly by a human observer showed greater FID than individuals approached more slowly, but also fled further (Cooper 2006). In response to being approached repeatedly, Schistocerca alutacea grasshoppers did not increase FID but fled farther, such that the pursuer failed to keep up with them (Bateman & Fleming 2014). Gryllus bimaculatus crickets can be stimulated to flee by a puff of air on the cerci from behind. A single puff causes a short run, but a continuous sequence of puffs causes sustained escape running and turning away from the stimulus source (Gras & Hörner 1992). Mosquito pupae Culex pipiens close to the surface of the water are presumably more at risk of aerial predation than ones that are deeper in the water column. When exposed to a simulated attack from above, they flee farther than ones that are initially deeper (Rodríguez-Prieto et al. 2006).
Increasing the DF comes with costs, since it removes the prey animal farther from its original position, which may have been optimal for the individual. Increasing DF must also come at a metabolic cost, and the energy used for locomotion will need to be later replaced through increased foraging.
7.2.2.2.3 Flight behavior
Flight can be augmented as an effective escape tactic by varying the angle of flight (Chapter 8). Assassin bugs Triatoma infestans consistently escape away from a stimulus at approximately 120°, which corresponds to the limits of their visual zone, thereby maximizing distance from the predator while still allowing the assassin bug to track it (Lazzari & Varjú 1990). However, repeatedly fleeing away from a predator on a set trajectory may result in anticipation of this escape response by the predator while, in some cases, completely random-direction fleeing may sometimes result in fleeing toward a predator (Card 2012).
Unpredictable, changing, flight patterns (termed “protean” behavior) can therefore be advantageous (Humphries & Driver 1970; Jones et al. 2011; Chapter 8). Cockroaches Periplaneta americana choose from a variety of escape trajectories that are at angles away from the threat (Domenici et al. 2008). On the approach of a predator model, ocean skaters Halobates robustus not only increase escape speed but also the frequency of turns on the water’s surface, performing protean turning and moving (Treherne & Foster 1981).
The grasshopper Psinidia fenestralis shows a bimodal pattern of escape trajectories, leaping either directly away from or at right angles to the observer’s approach path (Bateman & Fleming 2014) (Figure 7.3). When approached repeatedly, however, the grasshoppers more consistently use lateral escape routes. Protean flight and flight lateral to the predator’s trajectory may be farther enhanced by wing coloration in oedipodine grasshoppers (e.g., Dissosteira carolina, Cooper 2006; Oedipoda caerulescens, Kral 2010; Psinidia fenestralis, Bateman & Fleming 2014): the rapid disappearance of their colorful underwings upon landing may enhance subsequent crypsis. Performing “hook” landings (e.g., Oedipoda caerulescens; Kral 2010), such that they face back the way they came, makes it more difficult for predators to shift to a different kind of search image to find their prey, and also improves monitoring of the approaching predator. Interestingly, the cyrtacanthridine grasshopper S. alutacea, which has non-contrasting under-wings, tends to shift away from use of lateral escape paths with persistent pursuit, and rely more on longer escape flights directly away from the observer (Bateman & Fleming 2014). Paroxya atlantica grasshoppers that have undergone autotomy of a limb always escape from a disturbance laterally (Bateman & Fleming 2011).

Figure 7.3
Movement of four grasshopper species in relation to the trajectory of an approaching human observer. Angles of 180° indicate the animals moved directly away from the observer, angles around 90° indicate they moved away perpendicularly to the observer’s approach path; escapes to the left- and right-hand side of the approach path have been pooled. Each circular line shows one individual. Data for (a) to (c) clearly show a bimodal distribution of escape angles. Data for (b) to (d) were for the first approach of these individuals.
(Bateman & Fleming 2011; 2014; we calculated (d) from Cooper, 2006)
7.2.2.3 Retreating to cover
Some organisms that flee have the option of hiding in refuges (Chapter 9). Among invertebrates, some of the best studied taxa for this behavior are fiddler crabs that retire toward self-constructed burrows when approached by predatory stimuli (e.g., Hemmi 2005a,b; Wong et al. 2005; Hemmi & Pfeil 2010). Having entered a refuge, a prey organism must make an economic decision on when to emerge (Hugie 2003; Martín 2014).
Boldness in emerging from shelter can vary between and among populations and individuals (Chapter 15), and is influenced by risk assessment (Sih 1986). Fiddler crabs Uca mjoebergi show a correlation between “boldness” (indicated by emerging sooner) and higher aggression in other interactions, and bold and aggressive males ultimately have higher mating success (Reaney & Backwell 2007). Gryllus integer cricket males vary in calling-bout lengths, and males with longer, more conspicuous songs take longer to emerge from a shelter in a novel environment than do males with shorter songs (Hedrick 2000). Male G. bimaculatus that have undergone a simulated predatory encounter resulting in autotomy of a hind limb also show more caution in emerging from a shelter than do intact conspecifics (Bateman & Fleming 2014). The spider Agelenopsis aperta retreats down the funnel of its web when disturbed by large-amplitude vibrations of the web consistent with disturbance by a bird predator; latency to re-emergence to a foraging position on the web is greater in sites with higher risk of predation by birds (Riechert & Hedrick 1990).
Hermit crabs carry shells of other organisms into which they can retreat. Alternatively, they can flee. Pagurus acadianus spends a longer time hiding in its shell when it has been handled by predatory lobsters (Scarratt & Godin 1992). The type of shell used by hermit crabs can also influence economic decisions: exposure to the odor of a crushed conspecific had no effect on the choice of either of two host shells (one of which was more robust than the other) in P. filholi, but the presence of a predatory crab induced a preference for the more robust host shell (Mima et al. 2003). Additionally, although hermit crabs spent longer in the more robust shells than the more vulnerable shells, they reduced time spent in the both shells overall and resorted to fleeing from a predator stimulus in the presence of the predatory crab. Hermit crabs also seem to be able to assess their conspicuousness: P. bernhardus that have been induced to inhabit shells that are conspicuous against the substrate show much longer hiding times in these shells after disturbance than when in shells that are cryptic against the substrate (Briffa & Twyman 2011).
Although sessile organisms cannot flee, but only retreat to shelter, they will still face the economic decision of how long to remain in the refuge before re-emerging to feed. The polychaete worm Serpula vermicularis filter-feeds from the entrance of its self-constructed calcareous tube. As food in the water column tends to come in “pulses” rather than uniformly, time spent hiding in the tube can be costly. Variation in latency to emerge after a predatory stimulus (a mechanical shock) is, then, a relatively elaborate result of tracking short-term changes in food availability (even changes in food availability on a relative basis) through comparing current feeding conditions to those previously experienced (Dill & Fraser 1997). Similarly, the barnacle Balanus glandula, when induced to hide by a shadow simulating a predator, hid longer if they had been feeding prior to hiding (Dill & Gillett 1991).
Major costs of retreating to shelter are the cost of monitoring the environment to ensure it is safe before re-emerging (see also section 7.2.1; Chapter 9) and the cost of suspending other activities, particularly foraging. Once an organism has descended into a burrow, it cannot effectively monitor the predator: over 70% of Uca vomeris crabs approached tangentially by a dummy predator remain on the surface and monitor the predator, despite having retreated toward their burrows, and only descend into the burrow when the predatory stimulus approaches directly (Hemmi & Pfeil 2010).
Monitoring can potentially be disrupted by other stimuli. The hermit crab Coenobita clypeatus withdraws into its shell when a black object (the “predator” stimulus) looms toward it. If the crabs are exposed to the noise of a motor boat engine at the same time, they allow the looming stimulus to approach closer before withdrawing, implying that the noise obscures the stimulus or “distracts” the prey and ultimately makes it more vulnerable to predation (Chan et al. 2010).
7.3 Factors that influence invertebrate escape response
The EEM predicts that animals should vary their responses according to the risk presented by the situation, balancing the benefits of moving away with the costs incurred (Chapter 2). In this section, we discuss the moderating effects of certain intrinsic and extrinsic variables on escape behavior.
7.3.1 Food handling
In addition to the examples for sessile organisms (section 7.2.2.3), food handling can also influence the escape behavior of mobile invertebrates. In captivity, crayfish Procambarus clarkii that are manipulating large, immobile pieces of food have shorter FID and are less likely to try to escape from an approaching fish net than if they are feeding on small, portable pieces of food (Bellman & Krasne 1983). This finding is consistent with the prediction by the EEM that FID is greater for larger food items because the cost of fleeing increases as the amount of food left behind increases. Alternatively, it is possible that the net is not perceived as a “predatory stimulus,” but may actually be inducing behavior typical for crayfish avoiding competition with conspecifics, i.e., staying to guard immobile sources of food. By comparison, in a similar situation, FID of hermit crabs Pagurus acadianus (stimulated with real and model predatory lobsters) was not influenced by mass of a food item, but the crabs moved further away when they were carrying lighter food items (Scarratt & Godin 1992).
7.3.2 Group living
The drivers of group living in invertebrates may be much the same as in vertebrates, including enhanced predator detection through higher cumulative vigilance via the “many-eyes” effect, dilution of predation risk, and increased predator defense through behaviors such as bunching (Krause & Ruxton 2002). Group size is therefore likely to influence the economics of FID decisions.
Juvenile water striders Gerris remigis show an increase in FID with increasing group size when approached by potentially cannibalistic adults, which appears to support the “many-eyes” hypothesis (Dill & Ydenberg 1987). However, when the group size continues to increase, FID decreases. Although Dill and Ydenberg (1987) proposed that this response suggested a trade-off between fleeing and risk dilution as a factor of increased group size, predator detection might also be compromised in larger groups.
As their colony increases in size, colonial-dwelling spiders Metepeira incrassata have a reduced chance of falling victim to predatory wasps that attack a series of spiders in each raid, mainly through an “early-warning” effect due to web vibrations caused by the wasps (Uetz et al. 2002). The likelihood of spiders dropping from the nest increased later in the attack run of the wasps’ raid. Consequently, adult female spiders at the center of the nest had longer FID than did those on the edge of the nest due to this “early warning.” Interestingly, immature spiders (which are less preferred prey for the wasps) showed no difference in FID among positions in the colony.
Ocean skaters Halobates robustus approached by a predatory stimulus swiftly transmit avoidance behavior (speeding up and increasing frequency of turns) through their groups. This transmission is faster even than the speed of the approaching stimulus (Treherne & Foster 1981).
7.3.3 Effects of body size and age on escape behavior
Body size of prey in relation to the predator may influence escape responses for two reasons. Firstly, larger individuals may more effectively protect themselves by using other defenses (e.g., kicking, biting, struggling, faster running; Bateman & Fleming 2008). Secondly, performance traits may be related to size: e.g., jumping spiders Servaea incana can run and climb faster as body size increases, but endurance decreases, suggesting an evolutionary trade-off (McGinley et al. 2013). Additionally, changes in body size may influence more fundamental features, such as the suite of predators a prey species faces.
Consequently, we should predict ontogenetic differences in escape behavior. Following a puff of air (intended to simulate a predatory spider), juvenile wood crickets Nemobius sylvestris are more likely to jump than are adults, and juveniles also flee proportionally farther (Arai et al. 2007). Mayfly larvae Baetis tricaudatus that have detected predatory fish or stonefly larvae reduce their foraging activity and increase the likelihood of drifting out of dangerous patches (Scrimgeour & Culp 1994); the likelihood of drift in the presence of the dace was, however, reduced in large larvae, which could reflect higher risk assessment by smaller individuals (Scrimgeour et al. 1994). Smaller barnacles Balanus glandula hide longer than larger ones after a disturbance (Dill & Gillett 1991), possibly reflecting higher risk for smaller individuals.
7.3.4 Effects of temperature on escape behavior
Temperature influences all biochemical processes and therefore fundamentally influences the physiological responses of ectotherms. Rates of processes rise with increasing temperature before stabilizing and then falling as temperatures increase beyond thermal tolerance limits (Hochachka & Somero 2002). The contractile frequency and power output of muscle tissues depend greatly on temperature: the minimum flight temperature for the thoracic muscles of locusts to generate enough power for sustained flight is ~20°C (Chappell & Whitman 1990 and references therein). Under higher temperatures, tonic immobility in water scorpions Ranatra sp. is reduced (Holmes 1906). Scallops escape by jet propulsion of water through a valve and the clapping of the bivalve shell by the muscular mantle; higher temperatures generally result in higher valve contraction, and muscle contractile properties also increase (Guderley & Tremblay 2013). Indeed, the scallop Argopecten colbecki cannot swim at all at 2°C (Peck et al. 2004).
Crustaceans actively avoid and exit temperature zones that approach their tolerance limits and, when in the middle of their optimum range, they show reduced movement, which ensures remaining in the zone (Lagerspetz & Vainio 2006). Outside this zone, many species of crustacean show reduced ability to carry out reproductive behavior, perform phototactic behavior, right themselves, or show efficient escape reflexes; in some cases they even have reduced walking ability (see Lagerspetz & Vainio 2006 for a review). Barnacles withdraw their cirrae when the predatory stimulus of a shadow falls across them and this response is temperature dependent, occurring faster in the center of the optimum temperature range (Lagerspetz & Kivivuori 1970).
7.4 Conclusions and future research
Although there is a growing body of research that explores the economic decisions of escape behavior with invertebrate models, there are still many taxa that have not been examined. Each taxon promises its own unique insight into the costs and benefits of escape behavior according to the particular set of circumstances it faces. In particular, the different modes of communication (visual, acoustic, tactile, and chemical) are each likely to have different influences on antipredation behavior.
Despite the fact that one of the first empirical papers to explore the predictions of the EEM was on water striders (Dill & Ydenberg 1987), invertebrates are underrepresented in the literature since then. Given their great diversity, we encourage more researchers to consider invertebrates as model taxa for studies of escape behavior; they can be very useful for such studies, particularly considering the ease of manipulating them, the effects of autotomy and ontogenetic stages, and the ease of obtaining large sample sizes.
References
Arai, T., Tominaga, O., Seikai, T. & Masuda, R. (2007). Observational learning improves predator avoidance in hatchery-reared Japanese flounder Paralichthys olivaceus juveniles. Journal of Sea Research, 58, 59-64.
Bateman, P. W. & Fleming, P. A. (2005). Direct and indirect costs of limb autotomy in field crickets Gryllus bimaculatus. Animal Behaviour, 69, 151-159.
Bateman, P. W. & Fleming, P. A. (2006a). Increased susceptibility to predation for autotomized house crickets (Acheta domestica). Ethology, 112, 670-677.
Bateman, P. W. & Fleming, P. A. (2006b). Sex, intimidation and severed limbs: The effect of simulated predator attack and limb autotomy on calling behavior and level of caution in the field cricket Gryllus bimaculatus. Behavioral Ecology and Sociobiology, 59, 674-681.
Bateman, P. W. & Fleming, P. A. (2008). An intra-and inter-specific study of body size and autotomy as a defense in Orthoptera. Journal of Orthoptera Research, 17, 315-320.
Bateman, P. W. & Fleming, P. A. (2011). Failure to launch? The influence of limb autotomy on the escape behavior of a semiaquatic grasshopper Paroxya atlantica (Acrididae). Behavioral Ecology, 22, 763-768.
Bateman, P. W. & Fleming, P. A. (2013a). The influence of web silk decorations on fleeing behaviour of Florida orb weaver spiders, Argiope florida (Aranaeidae). Canadian Journal of Zoology, 91, 468-472.
Bateman, P. W. & Fleming, P. A. (2013b). Signaling or not-signaling: variation in vulnerability and defense tactics of armored ground crickets (Acanthoplus speiseri: Orthoptera, Tettigoniidae, Hetrodinae). Journal of Insect Behavior, 26, 14-22.
Bateman, P. W. & Fleming, P. A. (2014). Switching to Plan B: changes in the escape tactics of two grasshopper species (Acrididae: Orthoptera) under repeated predatory approaches. Behavioral Ecology and Sociobiology, 68, 457-465.
Bellman, K. L. & Krasne, F. B. (1983). Adaptive complexity of interactions between feeding and escape in crayfish. Science, 221, 779-781.
Ben-Ari, M. & Inbar, M.(2013). When herbivores eat predators: Predatory insects effectively avoid incidental ingestion by mammalian herbivores. PloS ONE, 8, e56748.
Briffa, M. & Twyman, C. (2011). Do I stand out or blend in? Conspicuousness awareness and consistent behavioural differences in hermit crabs. Biology Letters, 7, 330-332.
Camhi, J. M. (1969). Locust wind receptors I. Transducer mechanics and sensory response. Journal of Experimental Biology, 50, 335-348.
Card, G. M. (2012). Escape behaviors in insects. Current Opinion in Neurobiology, 22, 180-186.
Castellanos, I., Barbosa, P., Zuria, I., Tammaru, T. & Christman, M. C. (2011). Contact with caterpillar hairs triggers predator-specific defensive responses. Behavioral Ecology, 22, 1020-1025.
Chan, A. A. Y.-H., Giraldo-Perez, P., Smith, S. & Blumstein, D. T. (2010). Anthropogenic noise affects risk assessment and attention: The distracted prey hypothesis. Biology Letters, 6, 458-461.
Chappell, M. A. & Whitman, D. W. (1990). Grasshopper thermoregulation. In Chapman, R. F. & Joern, A. (eds.) Biology of Grasshoppers. New York: Wiley.
Cooper, W. E., Jr. (2006). Risk factors and escape strategy in the grasshopper Dissosteira carolina. Behaviour, 143, 1201-1218.
Cooper, W. E., Jr. & Frederick, W. G. (2010). Predator lethality, optimal escape behavior, and autotomy. Behavioral Ecology, 21, 91-96.
Corcoran, A. J., Wagner, R. D. & Conner, W. E. (2013). Optimal predator risk assessment by the sonar-jamming Arctiine moth Bertholdia trigona. PloS one, 8, e63609.
Dill, L. & Fraser, A. (1997). The worm re-turns: Hiding behaviour a tube-dwelling marine polychaete, Serpula vermicularis. Behavioral Ecology, 8, 186-193.
Dill, L. M. & Gillett, J. F. (1991). The economic logic of barnacle Balanus glandula (Darwin) hiding behavior. Journal of Experimental Marine Biology and Ecology, 153, 115-127.
Dill, L. M. & Ydenberg, R. C. (1987). The group size-flight distance relationship in water striders (Gerris remigis). Canadian Journal of Zoology, 65, 223-226.
Dill, L. M., Fraser, A. H. G. & Roitberg, B. D. (1990). The economics of escape behaviour in the pea aphid, Acyrthosiphon pisum. Oecologia, 83, 473-478.
Domenici, P., Booth, D., Blagburn, J. M. & Bacon, J. P. (2008). Cockroaches keep predators guessing by using preferred escape trajectories. Current Biology, 18, 1792-1796.
Dukas, R. (1998). Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making. University of Chicago Press.
Edmunds, M. (1974). Defence in Animals: A Survey of Anti-predatory Defences. Burnt Mill, Harlow: Longman.
Fleming, P. A. & Bateman, P. W. (2007). Just drop it and run: The effect of limb autotomy on running distance and locomotion energetics of field crickets (Gryllus bimaculatus). Journal of Experimental Biology, 210, 1446-1454.
Fleming, P. A., Muller, D. L. & Bateman, P. W. (2007). Leave it all behind: A taxonomic perspective of autotomy in invertebrates. Biological Reviews, 82, 481-510.
Gish, M., Dafni, A. & Inbar, M. (2010). Mammalian herbivore breath alerts aphids to flee host plant. Current Biology, 20, R628-R629.
Gish, M., Dafni, A. & Inbar, M. (2011). Avoiding incidental predation by mammalian herbivores: Accurate detection and efficient response in aphids. Naturwissenschaften, 98, 731-738.
Gras, H. & Hörner, M. (1992). Wind-evoked escape running of the cricket, Gryllus bimaculatus. I. Behavioural analysis. Journal of Experimental Biology, 171, 189-214.
Guderley, H. & Tremblay, I. (2013). Escape responses by jet propulsion in scallops. Canadian Journal of Zoology, 91, 420-430.
Gyssels, F. G. M. & Stoks, R. (2005). Threat-sensitive responses to predator attacks in a damselfly. Ethology, 111, 411-423.
Hassenstein, B. & Hustert, R. (1999). Hiding responses of locusts to approaching objects. Journal of Experimental Biology, 202, 1701-1710.
Hatle, J. D. & Faragher, S. G. (1998). Slow movement increases the survivorship of a chemically defended grasshopper in predatory encounters. Oecologia, 115, 260-267.
Hedrick, A. V. (2000). Crickets with extravagant mating songs compensate for predation risk with extra caution. Proceedings of the Royal Society of London Series B-Biological Sciences, 267, 671-675.
Hemmi, J. M. (2005a). Predator avoidance in fiddler crabs: 1. Escape decisions in relation to the risk of predation. Animal Behaviour, 69, 603-614.
Hemmi, J. M. (2005b). Predator avoidance in fiddler crabs: 2. The visual cues. Animal Behaviour, 69, 615-625.
Hemmi, J. M. & Pfeil, A.(2010). A multi-stage anti-predator response increases information on predation risk. Journal of Experimental Biology, 213, 1484-1489.
Hochachka, P. W. & Somero, G. N.(2002). Biochemical Adaptation: Mechanism and Process in Physiological Evolution. New York: Oxford University Press.
Holmes, S. J. (1906). Death-feigning in Ranatra. Journal of Comparative Neurology and Psychology, 16, 200-216.
Hugie, D. M. (2003). The waiting game: a “battle of waits” between predator and prey. Behavioral Ecology, 14, 807-817.
Humphries, D. A. & Driver, P. M. (1970). Protean defence by prey animals. Oecologia, 5, 285-302.
Javůrková, V., Šizling, A. L., Kreisinger, J. & Albrecht, T. (2012). An alternative theoretical approach to escape decision-making: the role of visual cues. PloS one, 7, e32522.
Jones, K. A., Jackson, A. L. & Ruxton, G. D. (2011). Prey jitters; protean behaviour in grouped prey. Behavioral Ecology, 22, 831-836.
Juanes, F. & Smith, L. (1995). The ecological consequences of limb damage and loss in decapod crustaceans: A review and prospectus. Journal of Experimental Marine Biology and Ecology, 193, 197-223.
Kral, K. (2010). Escape behaviour in blue-winged grasshoppers, Oedipoda caerulescens. Physiological Entomology, 35, 240-248.
Krause, J. & Ruxton, G. D. (2002). Living in Groups. Oxford University Press.
Lagerspetz, K. Y. & Vainio, L. A.(2006). Thermal behaviour of crustaceans. Biological Reviews, 81, 237-258.
Lagerspetz, K. Y. H. & Kivivuori, L.(1970). The rate and retention of the habituation of the shadow reflex in Balanus improvisus (Cirripedia). Animal Behaviour, 18, 616-620.
Lazzari, C. & Varjú, D. (1990). Visual lateral fixation and tracking in the haematophagous bug Triatoma infestans. Journal of Comparative Physiology A, 167, 527-531.
Lee, S.-I., Hwang, S., Joe, Y.-E. et al. (2013). Direct look from a predator shortens the risk-assessment time by prey. PloS ONE, 8, e64977.
Lewkiewicz, D. A. & Zuk, M. (2004). Latency to resume calling after disturbance in the field cricket, Teleogryllus oceanicus, corresponds to population-level differences in parasitism risk. Behavioral Ecology and Sociobiology, 55, 569-573.
Losey, J. E. & Denno, R. F. (1998). The escape response of pea aphids to foliar-foraging predators: Factors affecting dropping behaviour. Ecological Entomology, 23, 53-61.
Martín, J. & Pilar, L. (2014). Hiding time in refuge. In Cooper, W. E., Jr. & Blumstein, D. T. (eds.) Escaping from Predators: An Integrative View of Escape Decisions. Chapter 9.
McGinley, R. H., Prenter, J. & Taylor, P. W. (2013). Whole-organism performance in a jumping spider, Servaea incana (Araneae: Salticidae): Links with morphology and between performance traits. Biological Journal of the Linnean Society, 110, 644-657.
McPeek, M. A., Schrot, A. K. & Brown, J. M. (1996). Adaptation to predators in a new community: Swimming performance and predator avoidance in damselflies. Ecology, 77, 617-629.
Miller, L. A. & Olesen, J. (1979). Avoidance behavior in green lacewings. Journal of Comparative Physiology, 131, 113-120.
Miller, L. A. & Surlykke, A. (2001). How some insects detect and avoid being eaten by bats: Tactics and countertactics of prey and predator. BioScience, 51, 570-581.
Mima, A., Wada, S. & Goshima, S. (2003). Antipredator defence of the hermit crab Pagurus filholi induced by predatory crabs. Oikos, 102, 104-110.
Miyatake, T. (2001). Diurnal periodicity of death-feigning in Cylas formicarius (Coleoptera: Brentidae). Journal of Insect Behavior, 14, 421-432.
Nelson, M. K. & Formanowicz, D. R., Jr. (2005). Relationship between escape speed and flight distance in a wolf spider, Hogna carolinensis (Walckenaer 1805). Journal of Arachnology, 33, 153-158.
Ohno, T. & Miyatake, T. (2007). Drop or fly? Negative genetic correlation between death-feigning intensity and flying ability as alternative anti-predator strategies. Proceedings of the Royal Society B: Biological Sciences, 274, 555-560.
Peck, L. S., Webb, K. E. & Bailey, D. M. (2004). Extreme sensitivity of biological function to temperature in Antarctic marine species. Functional Ecology, 18, 625-630.
Ratcliffe, J. M., Fullard, J. H., Arthur, B. J. & Hoy, R. R. (2011). Adaptive auditory risk assessment in the dogbane tiger moth when pursued by bats. Proceedings of the Royal Society B: Biological Sciences, 278, 364-370.
Reaney, L. T. & Backwell, P. R. Y. (2007). Risk-taking behavior predicts aggression and mating success in a fiddler crab. Behavioral Ecology, 18, 521-525.
Riechert, S. E. & Hedrick, A. V. (1990). Levels of predation and genetically based anti-predator behaviour in the spider, Agelenopsis aperta. Animal Behaviour, 40, 679-687.
Rind, F. C. & Simmons, P. J. (1992). Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I. Selective responses to approaching objects. Journal of Neurophysiology, 68, 1654-1666.
Robinson, M. H., Abele, L. G. & Robinson, B.(1970). Attack autotomy: A defence against predators. Science, 169, 301-302.
Rodríguez-Prieto, I., Fernández-Juricic, E. & Martín, J. (2006). Anti-predator behavioral responses of mosquito pupae to aerial predation risk. Journal of Insect Behavior, 19, 373-381.
Rosen, M. J., Levin, E. C. & Hoy, R. R. (2009). The cost of assuming the life history of a host: acoustic startle in the parasitoid fly Ormia ochracea. Journal of Experimental Biology, 212, 4056-4064.
Scarratt, A. M. & Godin, J.-G. J. (1992). Foraging and antipredator decisions in the hermit crab Pagurus acadianus (Benedict). Journal of Experimental Marine Biology and Ecology, 156, 225-238.
Scrimgeour, G. J. & Culp, J. M. (1994). Foraging and evading predators: The effect of predator species on a behavioural trade-off by a lotic mayfly. Oikos, 71-79.
Scrimgeour, G. J., Culp, J. M. & Wrona, F. J. (1994). Feeding while avoiding predators: evidence for a size-specific trade-off by a lotic mayfly. Journal of the North American Benthological Society, 368-378.
Sih, A. (1986). Antipredator responses and the perception of danger by mosquito larvae. Ecology, 434-441.
Stoks, R. (1998). Effect of lamellae autotomy on survival and foraging success of the damselfly Lestes sponsa (Odonata: Lestidae). Oecologia, 117, 443-448.
Stoks, R. (1999). Autotomy shapes the trade-off between seeking cover and foraging in larval damselflies. Behavioral Ecology and Sociobiology, 47, 70-75.
Treherne, J. E. & Foster, W. A. (1981). Group transmission of predator avoidance behaviour in a marine insect: The Trafalgar effect. Animal Behaviour, 29, 911-917.
Uetz, G. W., Boyle, J., Hieber, C. S. & Wilcox, R. S. (2002). Antipredator benefits of group living in colonial web-building spiders: the “early warning” effect. Animal Behaviour, 63, 445-452.
Weyel, W. & Wegener, G. (1996). Adenine nucleotide metabolism during anoxia and postanoxic recovery in insects. Experientia, 52, 474-480.
Wong, B. B. M., Bibeau, C., Bishop, K. A. & Rosenthal, G. G. (2005). Response to perceived predation threat in fiddler crabs: trust thy neighbor as thyself?Behavioral Ecology and Sociobiology, 58, 345-350.
Zuk, M. & Kolluru, G. R. (1998). Exploitation of sexual signals by predators and parasitoids. Quarterly Review of Biology, 73, 415-443.