ESCAPING FROM PREDATORS An Integrative View of Escape Decisions (2015)
Part II Escape and refuge use: theory and findings for major taxonomic groups
IIb Escape decisions prior to pursuit
5 Reptiles
William E. Cooper, Jr.
5.1 Introduction
Predator-prey encounters matching the scenario of optimal escape theory begin in two ways. Upon detecting an approaching predator, lizards and other reptiles that are moving often stop, presumably reducing the probability of being detected by the predator. Those that are immobile remain still for the same reason as they monitor approaching predators. A large majority of studies of flight initiation distance (FID) and other escape variables have reported effects of predation risk factors, which are important because they affect the cost of not fleeing. Although fewer studies report effects of factors that impose opportunity costs (i.e., costs of not fleeing), much of what is known about these effects has been learned in studies of lizards. Effects of the initial fitness of lizards and other reptiles on escape behavior have been studied only indirectly.
Reptiles are one of the three taxonomic groups in which escape behavior has been studied most extensively, the others being birds and mammals (Stankowich & Blumstein 2005; Chapter 3; Chapter 4). Although advances in our understanding of phylogenetic relationships among major vertebrate taxa has removed crocodilians from Reptilia, and the placement of turtles on the vertebrate phylogenetic tree remains uncertain, the traditional taxonomic categories of reptiles are covered in this chapter, primarily because herpetologists continue to study these groups. Because little is known about escape decisions by turtles, crocodilians, and snakes, most of this chapter presents current knowledge about escape by lizards. Snakes constitute a major lizard clade, but their escape behavior is presented separately because they differ ecologically and morphologically from other lizards.
Factors affecting decisions about when and how far to flee are the main focus, including factors that affect predation risk and cost of fleeing. The direction of fleeing in relation to predators and refuges, and the selection of and entry into refuges are also discussed. Effect sizes are correlation coefficients taken from the original studies or calculated from P values and sample sizes or other statistics using the Meta-Analysis Calculator. Values are mean ± SE. Many citations have been omitted from the text, but are available in the electronic supplementary material (ESM 5.1) along with a table of effects, effect sizes, taxa, and sources (ESM 5.2). These are available at www.cambridge.org/9781107060548. Information about alternative escape strategies is presented in concluding sections for lizards and snakes.
5.2 Lizards
5.2.1 Predation risk factors that affect escape during approach
5.2.1.1 Position, habitat, and environmental factors
5.2.1.1.1 Distance to refuge, perch height, and direction to refuge
Many lizards escape by running to and entering refuges, most commonly trees, logs, crevices in rocks, and animal burrows (Cooper 1998a). Fleeing lizards may stop on the surface or enter a refuge; many individuals stop very close to a refuge, avoiding costs of refuge use if the predator does not attack. The distance to the nearest refuge and its direction with respect to the approaching predator determine projected arrival times of predator and prey at the refuge (Cooper 1997a; Kramer & Bonenfant 1997). Because arrival time increases as distance to refuge increases, risk of being captured at a particular FID increases as distance to refuge increases. Therefore FID is predicted by economic escape theory to increase as distance to refuge increases. If lizards farther from a refuge are more likely than those adjacent to a refuge to stop before entering a refuge, distance fled (DF) will be shorter than distance to a refuge, but is likely to be longer than for lizards closer to a refuge for two reasons. First, lizards that remain outside a refuge must move far enough from the predator to provide a margin of safety in the event of continued approach. Second, distance fled by lizards close to a refuge must often be less than the margin of safety.
Lizards closer to a refuge initially are expected to permit closer approach before fleeing and are more likely to enter a refuge than those farther from a refuge. The predator’s proximity when escape begins implies greater risk of being captured should the prey not enter a refuge. Therefore when distance to a refuge is shorter, the probability of entering a refuge is predicted to be greater.
In all of 17 studies of ten lizard species representing five families, FID increased as distance to refuge increased (Figure 5.1). The effect size (r = 0.49 ± 0.06, range 0.16-0.93) was intermediate. The primary determinant of the effect size is likely to be the degree of variation in distance to refuge among individuals. The influence of distance to refuge on FID may be somewhat stronger than indicated by the mean effect size because the range of distance to refuge was small in some cases (Cooper & Wilson 2007). In some species that occur at highly variable distances from refuge, the effect size is large (Anolis lineatopus: r = 0.93, Cooper 2006a; Egernia cunninghami: r = 0.90, Eifler 2001; and Leiocephalus carinatus: r = 0.87, Cooper 2007a).
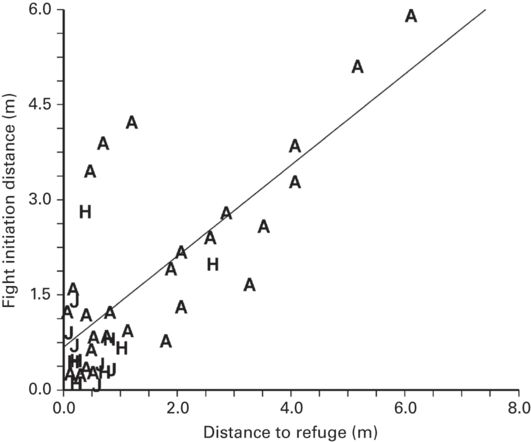
Figure 5.1
As distance to refuge increases, FID increases in Sceloporus woodi.
(Stiller & McBrayer 2013)
Two of the lowest r values were taken from a study in which the method of approach differed from that in all other studies (Bulova 1994). Bulova walked through the habitat in a straight line, and did not stop upon sighting a lizard, but continued on her path. Researchers in the remaining studies walked through the habitat until sighting a lizard, stopped moving, turned toward the lizard, and then walked directly toward it. In the only direct comparison of the effect size for the two methods, the effect sizes for Callisaurus draconoides were 0.16 in Bulova’s study and 0.47 in Cooper (2010a). Variation in directness of approach using Bulova’s method may have affected perceived risk, which is lower for indirect approaches. Because shorter FID is predicted for lower risk, the association between FID and distance to refuge would be weakened by including indirect approaches.
Distance to refuge is positively correlated with distance fled in all of ten studies of species from five families, significantly so in eight of them (r = 0.49 ± 0.09, range 0.12-0.91). Reasons for the large differences in effect size are not entirely clear, but include the difference in method of approach discussed above and interspecific differences in degree of variation in distance from refuge. In the only study to measure the relationship between distance to refuge and the probability of entering refuge when approached, a larger proportion of lizards entered refuge when closer than farther from refuge (r = 0.56, Cooper 2007a). The relationships of distance fled and probability of entering refuge to distance to refuge are as predicted by economic escape theory.
For lizards on tree trunks and other surfaces having vertical aspects, perch height strongly affects vulnerability to predation by terrestrial predators. Many species flee up trees to reach heights where they are safe and often flee to and along the side of the tree opposite that of the approaching predator, where their movements are invisible. Therefore perch height is expected to have effects on escape behavior similar to those of distance to refuge. For species that climb upward to escape, predation risk is greatest at perch height (PH) = 0. As a lizard climbs higher, its risk of predation decreases until it attains a height at and above which predation risk is zero and fleeing is unnecessary. Flight initiation distance should therefore decrease as PH increases in the range 0 ≤ PH ≤ PH0risk). Distance fled (climbed) should increase as PH decreases in the range 0 ≤ PH ≤ PH0risk, but should be unaffected at higher PH. When approached directly, all lizards at risk should flee before the predator reaches the tree.
Fewer species may flee downward from terrestrial or aerial predators. For example, grass-bush anoles (Anolis spp.) occupy low bushes that do not permit upward escape from terrestrial predators large enough to reach the tops of the bushes (Williams 1983). These species flee downward, often into low vegetation on the ground (Cooper 2006b, 2012). Other species escape upward when on vertical surfaces that are tall enough to permit escape, but flee downward or onto shorter vertical objects such as fence posts (Schneider et al. 2000). When prey flee downward, predation risk is lowest on the ground and increases as PH increases. Economic escape theory predicts that FID increases as PH increases when escape is downward. Distance fled also is expected to increase as PH above ground increases.
Predictions for FID have been confirmed consistently. In all of 13 species that flee upward, including representatives of five families, FID increased as PH decreased (Figure 5.2). The effect size is similar to that of distance to refuge (absolute value of r = −0.50 ± 0.07, range 0.12-0.88). For three species of Anolis that fled downward, FID increased as PH increased (r = 0.36 ± 0.13, range 0.27-0.50). Although the absolute magnitudes of the effect sizes for lizards that escape upward and downward are similar, the effects are in opposite directions. The escape responses of lizards that fled upward differed from those of species that escaped by fleeing downward (Fisher exact test, p = 0.0036, two-tailed). Distance fled decreased as PH increased in the sole species studied, which escaped upward. However, the relationship was weak and not significant. The range of PH in which some individuals flee and others do not presumably reveals individual differences in boldness or wariness (López et al. 2005).
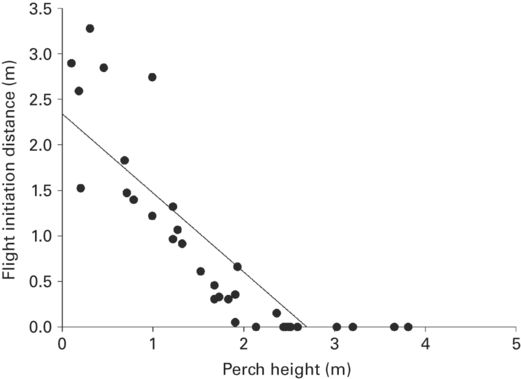
Figure 5.2
As perch height increases, FID decreases in Anolis graham.
(Cooper 2010b)
Prey sometimes flee to nearby refuges that may be in any direction, including in the direction of the predator. When a prey flees toward an approaching predator, it has less time to reach refuge ahead of the predator than when it flees away (Kramer & Bonenfant 1997). In one lizard species both FID (r = 0.55) and DF (r = 0.54) are longer when a prey flees to a refuge toward rather than away from the predator. The finding for FID matches the prediction based on greater risk of fleeing toward a predator. The longer DF for prey approaching predators suggests that the lizards may flee longer distances to reach secure refuges, flee all the way to refuge, or a higher proportion of the distance to refuge, when fleeing toward versus away from the predator, or that they flee toward the predator when alternative refuges are unavailable nearby. In another species when the predator is between the prey and refuge, FID is longer when the prey flees away from the predator (r = 0.62); lizards delay fleeing when the refuge is blocked, often fleeing toward, but around the predator at an acute angle (Cooper 1999a).
5.2.1.1.2 Microhabitat
Escape behavior may differ among microhabitats for multiple reasons, including differences in distance to refuge, detectability, and effects of substrate on running speed. Lizards that escape by climbing typically have a shorter distance to flee to safety when on trees than on other substrates, and are therefore predicted to have shorter FID and shorter DF. In the skink Plestiodon laticeps FID (r = 0.57 ± 0.05) and DF (r = 0.35 ± 0.03, range 0.32-0.37) were longer and likelihood of entering refuge was lower (r = 0.46 ± 0.02, range 0.44 - 0.48) for lizards on rocks and ground than on trees (r values for two studies each) (Cooper 1998a). Similar effects were observed in the phrynosomatidSceloporus virgatus (Cooper & Wilson 2007), for which FID (r = 0.79 ±.13, range = 0.65-0.92) and DF (r = 0.35 ± 0.09, range 0.26-0.45) were shorter for lizards on trees than on ground or rocks (two studies each). These findings are in part an extension of the results for perch height to situations in which prey must flee to a tree to attain zero PH.
5.2.1.1.3 Habitat openness and exposure
In habitats or microhabitats where lizards are more detectable or more vulnerable to attack, lizards are expected to be warier, which may be expressed by longer FID and DF, increased tendency to flee, and/or increased likelihood of entering refuge. Such effects have been investigated by comparing escape by lizards in habitats that differ in vegetative cover, when individuals in the same habitat are exposed versus partially concealed, in circumstances that affect their conspicuousness due to background matching and movement, and in microhabitats that differ in safety.
In open, sparsely vegetated habitats, prey may have longer FID and DF because they tend to be farther from refuges and are more detectable. Flight initiation distance was longer in open than densely vegetated habitats in four species; in two other species, FID was longer in open habitats in one of two studies. For all comparisons combined, the effect size is modest (0.37 ± 0.12), but quite variable (range 0.00-0.93), with only two values above 0.33.
Lizards fled longer distances in more open habitats in five of six comparisons in four species; in one of these species no effect was observed in one of two comparisons. The effect size for all comparisons was small (r = 0.25 ± 0.07). In a comparative study of 25 species of Liolaemus, DF was longer in more open than less open habitats (Schulte et al. 2004).
In all of six species from four families, FID was shorter for partially concealed than fully exposed lizards. The importance of partial concealment for risk assessment is suggested by the large effect size (r = 0.73 ± 0.05, range 0.54-0.85). In two species exposed lizards were more likely to flee than were partially concealed lizards (r = 0.57 ± 0.14, range 0.43-0.71).
5.2.1.1.4 Temperature
Body temperature strongly affects running speed in lizards (Huey 1982). At cold temperatures they are immobilized or move very slowly, but running speed increases as body temperature increases until high speed is maintained over a range of body temperatures near the preferred body temperature (Huey 1982). Because escape ability improves and risk when a predator is at a given distance decreases as running speed increases, prey are predicted to have shorter FID as body temperature increases. However, if cooler lizards stay closer to refuge or rely more on immobility to avoid being detected, FID might increase as body temperature increases or be unaffected by body temperature if the effects of temperature on speed and distance to refuge and/or crypsis via immobility counteract each other.
Few studies have reported effects of body temperature on escape behavior because lizards must be captured immediately after fleeing to measure body temperature, whereas air temperature and substrate temperature are easy to measure. Body temperature is more highly correlated with substrate temperature than with air temperature. Due to the weaker correlation of air and substrate temperature than body temperature with running speed (Hertz et al. 1983), predictions are less clear than for body temperature.
Flight initiation distance increased as body temperature decreased (Figure 5.3) in three species (r = 0.52 ± 0.10, range 0.39-0.72) and increased as body temperature increased in two other species (r = 0.44 ± 0.11). In two species the proportion of lizards that fled increased as body temperature increased (r = 0.92 ± 0.04, range 0.88-0.97). Flight initiation distance increased as substrate temperature increased in two studies of one species (r = 0.63 ± 0.09, range 0.54-0.72) and decreased as substrate temperature increased in another species (r = 0.39). Substrate temperature was uncorrelated with distance fled in two species.
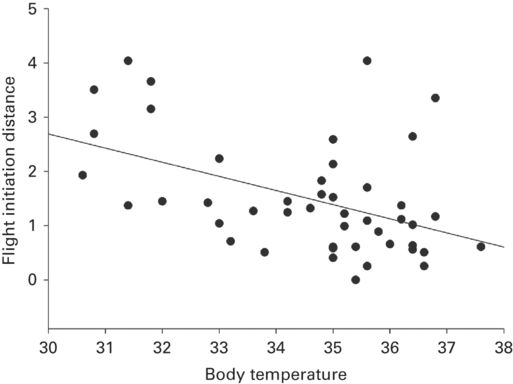
Figure 5.3
As body temperature increases, FID decreases in Sceloporus virgatus.
(Cooper 2011c)
Air temperature, being weakly related to body temperature, is often unrelated or weakly related to escape variables. In four species FID was unrelated to air temperature. In two species FID increased as air temperature increased (r = 0.16 ± 0.00, range 0.16-0.17), and in four species FID decreased as air temperature increased (r = 0.35 ± 0.09, range 0.21-0.57). In five of seven species, distance fled was unrelated to air temperature; in the other two species distance fled increased as air temperature increased (r = 0.22 ± 0.06, range 0.16-0.27). The proportion of lizards that fled increased at air temperature increased in a single species (r = 0.79). The proportion of individuals that entered refuge decreased as air temperature increased in all four studies of three species (r = 0.65 ± 0.15, range 0.28-1.0).
As expected, effect sizes for air temperature were smaller than for body temperature and substrate temperature. Temperature profoundly affects many aspects of behavior in ectotherms such as lizards, including all four escape variables examined. As suggested above, differences among species in the direction of the relationships of temperature to FID and DF may indicate that some species may stay closer to refuges when cool, whereas others do not adjust distance to refuge to current temperatures.
5.2.1.1.5 Wind speed
Bulova (1994) reasoned that high wind speed reduces body temperature, which in turn reduces running speed. She predicted that FID and DF would increase as wind speed increased to compensate for slower running. In one of two species in her study, FID decreased as wind speed increased (r = - 0.13), but wind speed did not affect DF. In the other species FID was unrelated to wind speed, but DF increased as wind speed increased (r = 0.26). These small effects might be indirect effects of environmental correlates of wind speed. That the direction of the effect of wind speed on FID was opposite to that predicted hints that lizards might be harder to detect in windy conditions.
5.2.1.1.6 Time of day and season
Time of day affected neither FID nor DF in the agamid Lophognathus temporalis (Blamires 1999). Presumably, behavioral thermoregulation suffices to maintain body temperature in a narrow range throughout the day, obviating any effect of diel variation in temperature on running speed.
5.2.1.2 Prey traits
5.2.1.2.1 Prey speed, morphology, and body condition
Because faster prey can reach refuge sooner or outrun predators, FID is predicted to decrease as prey speed increases. Distance fled is predicted to increase as running speed increases provided that lizards often flee without entering refuge and running speed does not affect duration of fleeing. Using morphological correlates of running speed, the prediction for FID was verified for one test and contradicted for another test in the same species (Hawlena et al.2009). That FID increased as tail length decreased (r = 0.34) is readily interpretable as an effect of decreasing running speed as tail length decreases (see section 5.2.1.2.4). However, FID increased as relative hind limb length increased (r = 0.48), contradicting the predicted effect of longer hind limb length to increase running speed. Hawlena et al. (2009) noted that running speed may be unimportant for escape by Podarcis lilfordi, which historically experienced very low predation pressure before human beings arrived in the Balearic Archipelago and currently experience very little predation in the population studied. These lizards typically stay close to vegetation that provides cover. Additional studies are needed to directly examine the effect of running speed on FID in lizard populations subject to higher levels of predation.
Morphological correlates of running speed and stride length were examined by Losos et al. (2002) in 17 cordylid species. Distance fled increased as femur length increased (r = 0.76) and as spine length increased (r = 0.75). These large interspecific effects are consistent with the prediction that DF increases as running speed increases.
Prey in better body condition have greater expected fitness than those in poorer condition. The asset protection principle states that prey with larger assets will be more cautious in their defense (Clark 1994). Escape theory therefore predicts that prey having better body condition have longer FID. In a single species FID increased as body condition improved (r = 0.57), as predicted. This test confirms the prediction for initial fitness, one of the three major components that determine FID in optimal escape theory (Cooper & Frederick 2007). Further tests of indicators of fitness are needed to assess the general validity of the prediction.
5.2.1.2.2 Habituation
When potential predators are present frequently or for long periods, but do not attack, prey may assess the threat of predation to be low, i.e., they may become habituated. Lizards in populations frequently exposed to people are predicted to have shorter FIDs than in populations infrequently exposed. Even if predation risk is high, prey that are constantly exposed to predators must accept greater risk if they are to perform essential activities such as foraging and social behavior. Both risk allocation (Lima & Bednekoff 1999) and habituation predict that FID will be shorter where predators are frequently or constantly present. Nevertheless, habituation is the likely explanation for decreased FID when the predators rarely or never attack because predation risk is very low, which is not the case under the risk allocation hypothesis, and because some prey become so habituated that they allow themselves to be touched (Cooper, personal observations).
In all of eleven lizard species from six families, FID was shorter in habituated than unhabituated populations (Figure 5.4). The effect size of habituation was substantial (r = 0.50 ± 0.06) and variable (range 0.16-0.73). It is expected to vary with the difference in degree of exposure to human beings among pairs of populations. All findings are consistent with the interpretation that FID is shorter in populations where lizards are habituated to people.
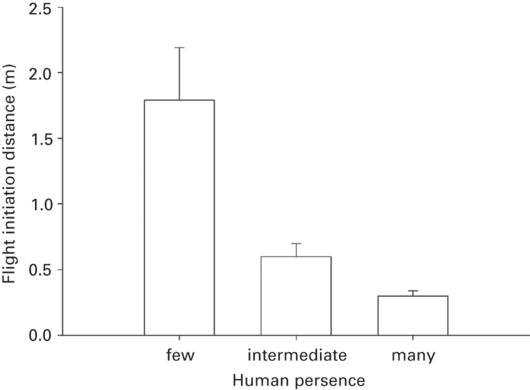
Figure 5.4
In Anolis lineatopus, FID is shorter where lizards are habituated to human presence.
(Cooper 2010b)
5.2.1.2.3 Prey sex, age, and body size
Economic escape theory makes no general predictions about sex differences. However, if ecological or morphological differences between sexes affect predation risk, the sex at greater risk is predicted to be warier. Flight initiation distance has been reported separately for the sexes in 38 species from eight families. Flight initiation distance was longer in males than females in eight species and in one of three populations in a ninth (r = 0.30 ± 0.08, range 0.20-0.42 for cases in which a difference was detected), whereas FID was longer in females than males in three species and in one of six populations of a fourth species (r = 0.22 ± 0.06, range 0.08-0.35). Males had longer FID than females in all three lacertid species studied (Figure 5.5), but no other relationships between families and occurrence of sex differences in FID are apparent. In 66% of 38 species, no sex difference in FID was observed.
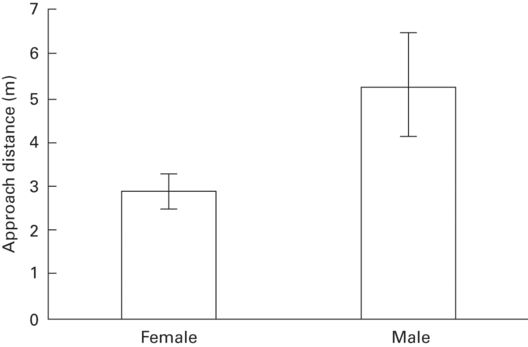
Figure 5.5
In Platysaurus intermedius, approach distance (FID) is longer in males than females.
(Lailvaux et al. 2003)
Distance fled did not differ between sexes in 21 species, and was longer in males than females in one species (r = 0.29) plus one of three populations of a second species (r = 0.42). In no case was DF longer for females than males. The proportion of individuals that entered refuges did not differ between sexes in five species. Males were more likely than females to enter refuges in one species and one of three populations of another (r = 0.39 ± 0.04); in no cases were females more likely to enter refuges.
The sexes of most lizards do not differ substantially in FID, DF, or refuge entry, but when sex differences occur, males seem more likely than females to be the warier sex, perhaps due to greater predation risk associated with some combinations of bright coloration, territorial patrolling and defense, and courtship behavior. However, social costs of fleeing may have countervailing effects (section 5.2.2.2), suggesting that greater wariness by males than females may occur when males are more active and exposed, but not in contexts in which fleeing imposes social opportunity costs. Greater wariness by females could be related to body size differences and slowing due to gravidity or pregnancy (Shine 1980; Cooper et al. 1990; Olsson et al. 2000). The effect of clutch or litter mass on escape behavior is less clear because gravid females may compensate by remaining closer to refuge or may rely more on crypsis due to immobility to avoid being detected (Cooper et al. 1990), which may explain the shorter FID of gravid than non-gravid Eulamprus tympanum (Schwartzkopf & Shine 1992).
Relationships between predator and prey sizes and predation risk are variable. As hatchlings grow, they may become too large to be prey of small predators. In such cases, hatchlings might be warier than larger, older individuals. For larger predators, lizards may become increasingly attractive prey as they grow larger. Furthermore, bright tail coloration in juveniles affects both detectability and escape ability (Cooper &Vitt 1985). The balance of these opposing effects may determine the relationship between body size and wariness (Cooper 2011a). Therefore information on such factors is needed to predict the relationships between size/age and wariness.
In studies with a limited range of lizard body size, neither FID (three species) nor DF (two species) was correlated with snout-vent length. However, prey size is important when a wider range of size is included. Larger lizards had longer FID for ten of eleven species belonging to five lizard families (r = 0.40 ± 0.09). In one of these ten species, Amblyrhynchus cristatus, a second study reported that FID is longer in juveniles than in either hatchlings or adults (r = 0.16), hinting that in this population, juveniles may remain attractive as prey to small predators and have become larger enough to be attractive to larger predators. Given the preponderance of cases in which FID is longer in older, larger, lizards, assessed risk when approached by a large predator appears to be greater for larger lizards. In the sole remaining species, FID did not differ among age/size categories.
For distance fled and refuge entry, age/size relationships to FID are variable. In three species no age/size differences were detected in DF. Of the two other species tested, DF was longer in adults than juveniles in one (r = 0.56) and longer in juveniles than adults in the other (r = 0.67). The proportion of lizards that entered refuge did not differ among age/size groups in one species; in two species it was greater for larger lizards (r = 0.49 ± 0.19) and in one species it was greater for hatchlings than adults (r = 0.10). The relationships of the escape variables DF and refuge entry with age and size may be affected by relationships of age/size with conspicuousness, vulnerability, and distance to refuge, but these factors remain to be investigated.
5.2.1.2.4 Autotomy and tail condition
Caudal autotomy (voluntary severing of the tail) often permits lizards to escape when contacted by a predator (Congdon et al. 1974; Cooper & Vitt 1985, 1991). Depending on how much of the tail is lost, the ability to use autotomy to escape in future encounters is diminished or lost until the tail regenerates. Running speed decreases after autotomy in most species (Bateman & Fleming 2009; Cooper et al. 2009a). Recently autotomized lizards are at greater risk when approached, and are predicted to adjust their behavior to reduce risk. One way of doing so is to increase FID. Additional possibilities are staying closer to refuge and entering refuge.
Effects of recent experimentally induced autotomy have been studied in the field in three species. In Cordylus melanotus autotomy did not affect FID or DF, but autotomy also did not affect running speed (McConnachie & Whiting 2003). In Holbrookia propinqua, FID was not affected by autotomy, but autotomized lizards stayed closer to refuges (r = 0.49) and males, but not females, fled shorter distances than intact lizards (r = 0.39; Cooper 2003). In Sceloporus virgatus autotomized individuals had longer FID (r = 0.58) and were more likely to enter refuges (r = 0.45) than individuals having intact tails (Cooper 2007b; Cooper & Wilson 2008).
Effects of autotomy on escape behavior are expected to wane over time as regeneration proceeds, but may persist longer if lizards have adjusted escape strategy to reduce the heightened risk. In observational studies some tail breaks have occurred recently, but others may have occurred weeks or months before observations. In such studies of six species from four families, FID was shorter in autotomized than intact lizards in four species (r = 0.40 ± 0.02), and in larger, but not smaller individuals in a fifth species (r = 0.68; Kelt et al. 2002). In the remaining species, intact and autotomized lizards had similar FIDs. This suggests that the long-term effect of autotomy on FID may be more consistent across taxa than the short-term effect. The long-term effect may be a consequence of staying closer to refuge or increased reliance on immobility to avoid detection.
Initial and prolonged effects of autotomy may differ. In Sceloporus virgatus FID is longer in recently autotomized than intact lizards (Cooper 2007b: Figure 5.6), but shorter when observations are made at longer intervals after autotomy. The initial effect may occur before adjustments in escape strategy occur.
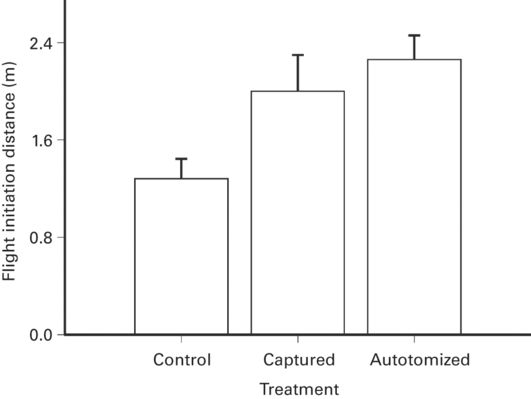
Figure 5.6
The FID in Sceloporus virgatus is longer after the tail is lost by autotomy that in lizards having intact tails.
(Cooper 2007b)
5.2.1.2.5 Female reproductive condition
Female lizards run slower when gravid or pregnant than at other times because their mass is increased by eggs or due to changes in physiology while gravid (Shine 1980; Cooper et al. 1990; Olsson et al. 2000). Because predation risk is greater for slower prey, gravid females are predicted to have longer FID unless they stay closer to refuge or rely more on crypsis conferred by immobility to avoid being detected. Flight inititation distance was shorter for gravid than non-gravid females in three species (r = 0.30 ± 0.06, range 0.18-0.36). In a fourth species FID did not differ between gravid and non-gravid females. Distance fled was shorter for gravid than non-gravid females in one species (r = 0. 23); in two other species gravidity did not affect DF. Two of the species for which neither FID nor DF was related to gravidity are ecologically similar congeners that usually remain near refuges (Smith 1996; Cooper 2011a). In one of those two species, the proportion of lizards that entered refuge also did not differ between gravid and non-gravid females. That FID was shortened in gravid females in three species and DF in one species suggests that compensatory changes in behavior during gravidity outweighed any increase in risk due to slower running speed. Where no significant differences were detected for FID or DF, gravid females may not have been greatly slowed or any increase in risk was balanced by changes in behavior that reduce risk.
5.2.1.2.6 Conspicuousness
Prey that are camouflaged, employ special resemblance to objects, or use cryptic postures (Figure 5.7) are predicted to permit closer approach than more conspicuous lizards. Because movement makes prey highly detectable, prey are predicted to have longer FID after recently moving than after prolonged immobility. Flight initiation distance was shorter where lizards matched backgrounds or objects (Figure 5.8) in two species (r = 0.56 ± 0.00). The horned lizard Phrynosoma modestum had longer FID after recent movement than after prolonged immobility (r = 0.82; Cooper & Sherbrooke 2010) and when standing than when lying flat on the ground because the contours of the lizard blend into the substrate when lying flat (r = 0.80; Cooper & Sherbrooke 2012). In Callisaurus draconoides FID and DF were longer after recent movement than immobility (Cooper & Sherbrooke 2013a). These are large effects that deserve further investigation as potentially important cues for risk assessment.

Figure 5.7
The round-tailed horned lizard, Phrynosoma modestum, adopts a posture that makes it resemble a small rock.
(Cooper & Sherbrooke 2010)
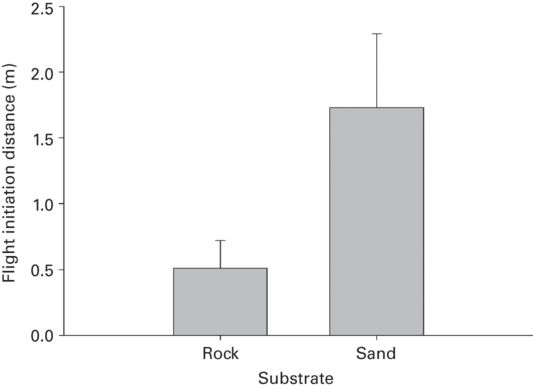
Figure 5.8
When among small rocks P. modestum permits closer approach before fleeing than when resting on open sand.
5.2.1.2.7 Pursuit-deterrent signaling
Because a predator that has received pursuit-deterrent signals is less likely to attack (Holley 1993; Ruxton et al. 2004), predation risk is reduced. In Callisaurus draconoides, FID of lizards that have performed a tail-waving display is shorter than in those that have not (r = 0.57, Cooper 2011b). This finding confirms the prediction that signaling decreases assessed risk based on the assumption that signaling must be somewhat effective to be maintained by natural selection.
5.2.1.3 Predator and approach factors
5.2.1.3.1 Predator approach speed
The predator’s approach speed strongly affects FID. Flight initiation distance was longer for faster than slower approach speed (Figure 5.9) in all seventeen studies of thirteen species representing eight families (r = 0.72 ± 0.04, range 0.30-0.94). These findings emphatically confirm the prediction of economic escape theory that FID increases as predation risk increases. When a predator approaches slowly, lizards allow closer approach because they have more time to reach a refuge than if a predator approaches more rapidly. Even for prey that flee across ground and use refuges infrequently, faster approach speed requires earlier fleeing to attain high escape speed before being overtaken.
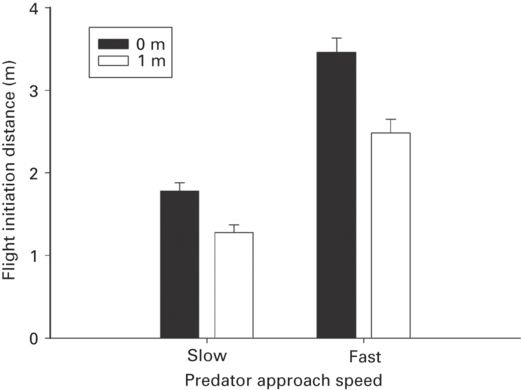
Figure 5.9
In Sceloporus jarrovii, FID is longer for direct approaches (0 m) at both slow and fast approach speeds than for indirect approaches that bypass the lizards by 1 m.
(Cooper & Avalos 2010)
Distance fled in two studies was longer when approach was faster (r = 0.57 ± 0.19). The proportion of lizards that entered refuge before ceasing to flee was greater for faster than slower approaches in two studies (r = 0.65 ± 0.02). For both DF and refuge entry, economic predictions are affected by the lizard’s distance from refuge. For individuals that flee into refuge or stop by the refuge’s entry point, DF is greater for lizards that are farther from the refuge prior to fleeing. The strength of the relationship between distance to refuge and distance fled must increase as the proportion of individuals that enter refuge increases.
Lizards adjacent to refuges are likely to enter upon fleeing, but many individuals at longer distances from refuge flee towards them, but stop fleeing before entering it. This is a consequence of the method of approach used in lizard studies: the approaching researcher stops moving as soon as a lizard begins to flee. After the predator stops, some prey stop before reaching refuge, thereby avoiding costs of refuge use. Lizards farther from refuge are more likely to stop fleeing without entering than lizards closer to refuges, resulting in a decrease in proportion of individuals that enter refuge as initial distance from refuge increases. Therefore the probability of entering refuge decreases as distance to refuge increases, whereas DF increases as distance to refuge increases (section 5.2.1.1.1).
Because FID and DF increase with distance to refuge, studies of effects of approach speed and other risk factors must avoid bias caused by differences in distance to refuge. In typical experimental studies, distance to refuge varies among individual prey, but average distance to refuge is not expected to vary among groups. The effect of variation in distance to refuge is to increase error variance, but not to bias results. This error variance can be reduced by restricting observations to a limited range of distance to refuge. Another way to detect an effect of approach speed is to statistically account for the effect of distance to refuge using multiple regression or by using distance to refuge as a covariate.
5.2.1.3.2 Directness of approach
A predator approaching indirectly, i.e., along a path that will not contact the prey, poses less threat than a predator approach directly along a line that intersects the prey’s position. An indirectly approaching predator is more likely than one approaching directly to have not yet detected and to fail to detect the prey before the prey passes out of the predator’s visual field. A directly approaching predator that has detected the prey is more likely to be approaching to attack, whereas an indirectly approaching predator is more likely to be searching for prey or passing through the area for other reasons.
The main variable used to indicate directness of approach is minimum bypass distance, the predator-prey distance when the predator reaches the closest point to the prey on its path. Minimum bypass distance is zero for direct approaches and is progressively longer as approach becomes less direct. For a fixed minimum bypass distance in such tangential approaches, the angle between the direct and indirect approach paths increases as starting distance decreases. It is important to use a fixed SD across trials unless the goal is to discern an effect of the angle of approach from that of the minimum bypass distance. To my knowledge, no studies have been done to make this potentially important distinction. Several studies have employed the same SD for direct approaches and relatively small minimum bypass distances. They have necessarily used longer SDs to permit longer minimum bypass distances. Reported effect of directness of approach may be a combination of effects of minimum bypass distance and approach angle.
Because risk is predicted to increase as approach becomes more direct, FID is expected to be longest for direct approach and to decrease when approach is less direct. This poses an experimental problem because when the minimum bypass distance exceeds the FID observed for direct approach, lizards do not flee. The minimum bypass distance for lizards that do not flee is therefore not useful as a maximum estimate of FID. For each species, pilot trials must be conducted to determine FIDs for direct approaches and a shorter minimum bypass distance must be chosen for study. Bypass distances longer than the FID for direct approach may be useful in studies of probability of fleeing, but are irrelevant for study of the effect of directness of approach on FID. Due to the effect on assessed risk, DF and probability of fleeing also are predicted to increase as directness of approach increases.
For all of eighteen species of lizards from nine families, FID was longer during direct than indirect approaches (Figure 5.9) using short minimum bypass distances (1-3 m). The longer minimum bypass distances were used for larger or warier species. The effect size was r = 0.44 ± 0.06, range 0.16 - 0.93).
The proportion of individuals that fled was greater for direct than indirect approaches in all of ten species (r = 0.70 ± 0.04, range 0.19-0.74). During indirect approaches, most individuals that fled did so only when the predator reached the minimum bypass distance (Cooper 1997b). One interpretation is that fleeing frequently occurs at the minimum bypass distance as the risk is greater at that distance than at any other distance. Between the starting point of approach and the point of minimum bypass distance, risk increases should the predator change direction and attack. Probability of fleeing should increase as the predator approaches the point closest to the predator. This presumably accounts for some variance in FID and probability of fleeing. However, the high frequency of fleeing only at the minimum bypass distance suggests another factor. Once the predator passes the closest point to the prey, risk diminishes so that fleeing is not necessary. The prey passes out of a human investigator’s line of sight at the minimum bypass distance. Therefore prey that flee at the minimum bypass distance may have delayed escape while the predator was close and could attack upon observing the prey’s movements. Once the predator cannot see a prey, the prey can safely withdraw to a nearby refuge (Cooper 1997b).
Little is known about the effect of directness of approach on DF, but DF was longer during direct than indirect approach in two studies. The effect size of directness on DF (r = 0.35 ± 0.07, range 0.28-0.42) was only half as large at its effect on refuge entry. The smaller effect on DF may be a consequence of proximity to refuge of numerous individuals in these studies.
Flight initiation distance, distance fled, and proportion of individuals that fled increase as the directness of approach increases, as predicted. For approaches with longer minimum bypass distances, probability of fleeing decreases to zero as minimum bypass increases. For minimum bypass distances greater than or equal to that for which no lizards flee, FID and DF also equal zero.
5.2.1.3.3 Predator turn direction
A predator that has stopped moving near a prey poses a threat that is reflected in the effect of standing distance on latency to flee (Chapter 2). If the predator’s body is oriented parallel to the prey’s body rather than facing it at a right angle, the predator may turn toward or away from the prey. When the predator rapidly turns toward the prey, assessed risk is expected to increase because the turn may indicate that the predator has detected the prey and is attacking. When a predator turns away from the prey, assessed risk may decrease because the predator may be paying attention to something else.
Risk while the predator is immobile increases as predator-prey distance decreases. When a predator begins to turn rapidly, the assessed risk is expected to increase. At long predator-prey distances, assessed risk will be too low to elicit flight regardless of the direction turned by the predator. When the predator is standing so close that risk is very high if it attacks, the prey may flee regardless of turn direction because it must react immediately to the movement without taking time to ascertain the direction of turning to the movement if it is to escape. It flees even if the predator turns away. The most interesting case occurs at intermediate predator-prey distances that allow the prey enough time to recognize the turn direction. In this range of distances, it may be predicted that the prey is more likely to flee when the predator turns toward than away from it.
These predictions have been verified consistently. At the shortest standing distances virtually all lizards flee immediately regardless of turn direction; at very long standing distances almost no lizards flee regardless of turn direction. Here the results are reported only for intermediate distances where turn direction affects probability of fleeing. For each species it is necessary to conduct pilot tests to determine the appropriate range of distances.
For each of ten lizard species from five families, the proportion of individuals that fled at the intermediate standing distance was greater when the predator turned toward than away from the prey (r = 0.64 ± 0.06, range 0.40-1.00; Figure 5.10). Clearly, lizards assess risk as being greater when a predator turns toward them than away. Two studies reported that the probability of fleeing, regardless of turn direction, increases as distance between the prey and immobile predator decreases (r = 0.69 ± 0.22). These findings confirm the predictions that the proportion of individuals that flee increases as the predator’s standing distance decreases and is greater when the predator turns toward the prey in an intermediate range of distances.
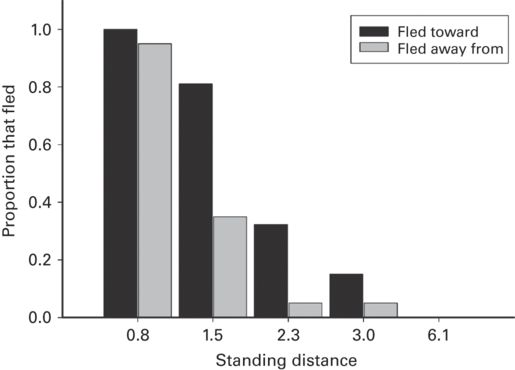
Figure 5.10
When a predator standing nearby suddenly turns, the proportion of Holbrookia propinqua that flee does not differ with turn direction for the shortest and longest standing distances, but is higher when the predator turns toward than away from the prey at intermediate distances.
(Cooper 1998c)
5.2.1.3.4 Approach elevation and sidedness
When prey are situated on slopes, predators may attack from above or below. If lizards interpret attack from above by a researcher as an aerial attack, they might assess risk as being greater due to the high expected speed of aerial predators. They also might be more vulnerable when attacked from above because lizards can escape by running up slopes where larger predators and investigators may be slowed. In a skink and a lacertid, FID was greater for approaches from above than below (r = 0.35 ± 0.10, range 0.26-0.45). Distance fled was greater for approaches from above in the lacertid (r = 0.24), but did not differ in the skink. The proportion of lizards that entered refuge did not differ between approached from above and below in the lacertid.
Because many species have side preferences (lateralization) and associated differences in reaction time (Ward & Hopkins 1993; Bisazza et al. 1998), escape responses might differ when lizards are approached from the left or right. However, in the sole study of this possibility, FID, DF, and refuge entry were unaffected by the side from which a predator approached (Cooper & Pérez-Mellado 2011).
5.2.1.3.5 Repeated approach and previous captures
If a predator approaches, the prey flees successfully, but the predator approaches again after a brief interval, the predator’s persistence indicates that it may pose an ongoing threat associated with greater risk of predation than a predator that approaches once and then desists. Therefore economic escape theory predicts that repeated approach should elicit longer FID; its extensions predict that DF and probability of entering refuge are less for initial than subsequent approaches.
In twelve species from four families, FID was longer for the second of two approaches (Figure 5.11). This difference was significant in 11 of 12 species with substantial effect size (r = 0.60 ± 0.04, range 0.00-0.84). Distance fled was greater in three species from three families (r = 0.55 ± 0.04, range 0.47-0.61). The proportion of individuals that entered refuge in three species from two families was greater for second than first approaches (r = 0.55 ± 0.09, range 0.47-0.72). All three predictions have been verified, demonstrating that prey update their assessment of predation risk to account for ongoing aspects of predator behavior during encounters.
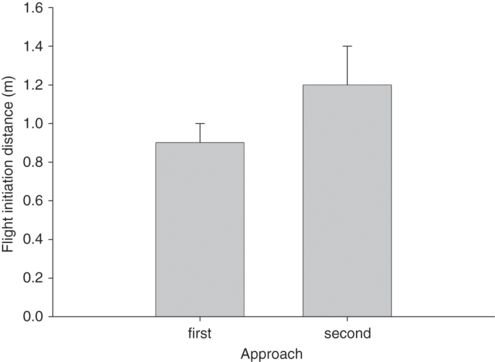
Figure 5.11
In Sceloporus virgatus FID is longer for the second of two successive approaches at a fast, but not a slow, approach speed.
(Based on data from Cooper 2009c)
Lizards that have been captured previously by researchers are expected to assess approach as riskier and to exhibit longer FID and DF. In three species FID increased as number of previous captures increased or was greater for previously captured than naïve prey (r = 0.37 ± 0.02, range 0.34-0.40). Distance fled was longer in one species for lizards that had been captured previously than had not (r = 0.34), but the proportion of individuals that entered refuge was not affected by previous capture.
5.2.1.3.6 Starting distance
Because the distance and duration moved by a predator during its approach can be a cue to increasing risk and can affect the ability of prey to obtain benefits while monitoring the predator, FID is predicted to increase as SD increases (Chapter 2; Cooper & Blumstein 2014). In birds and mammals FID typically increases as SD increases (Blumstein 2003; Stankowich & Coss 2007; Williams et al. 2014) as predicted, but findings for lizards have been mixed.
The relationship between SD and FID have been studied in seven species from five families. Among these are five species of ambush foragers that have very low rates of spontaneous movement and low cost of monitoring because feeding attempts are infrequent. When these lizards were approached at a slow walking speed, FID was not correlated with SD. When approached at a faster walking speed, FID increased as SD increased in one species (r = 0.50), but not another. The other two species are active foragers that have higher rates of spontaneous movement and spend a longer proportion of the time moving (Cooper 2007c). Of these, FID increased as SD increased at a fast, but not a slow, approach speed in the lacertid Podarcis lilfordi (Cooper et al. 2009b; Figure 5.12); it increased as SD increased at a slow approach speed in the teiid Aspidoscelis exsanguis (r = 0.42; Cooper 2008). These limited data suggest that the FID-SD relationship is stronger when predation risk is higher (at faster approach speed). This is consistent with (1) a dynamic increase in assessed risk related to distance approached by the predator that only occurs at a rapid approach speed in some species and (2) a stronger relationship exists between FID and SD in active foragers than ambush foragers. One reason for the latter effect may be that it is more costly for active than ambush foragers to remain motionless, which prevents active foraging, implying that the cost of monitoring may be greater (Cooper & Blumstein 2014) for active foragers. Starting distance was unrelated to DF in two species and to probability of entering refuge in one.
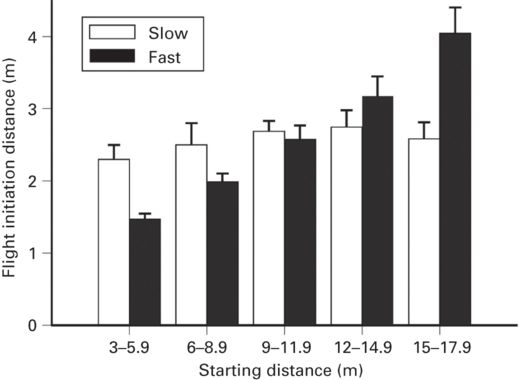
Figure 5.12
At fast, but not slow, approach speed, FID by Podarcis lilfordi increases as SD of the predator’s approach increases.
(Cooper et al. 2009b)
5.2.1.3.7 Sudden shadowing
A shadow that suddenly passes overhead may be a strong cue to attack by a predator, especially an aerial or large predator. Immediate escape is predicted when a shadow rapidly covers a lizard, and the effect on escape frequency should be greater when the shadow falls directly on a lizard than nearby, greater when the shadow’s speed is greater, and greater when a lizard is oriented horizontally where it is more vulnerable to attack from above than when oriented vertically (Cooper 2009a). When shadows were cast by a human hand, the proportion of lizards that fled was greater when the shadow fell directly on lizards than nearby (Figure 5.13) in three species (r = 0.76 ± 0.09, range 0.58-0.89). In single experiments the proportion of lizards that fled was greater when shadow speed was faster (r = 0.77) and when lizards were horizontally rather than vertically oriented (r = 0.82). Rapid shadowing appears to be a major cue to immediate threat of predation.
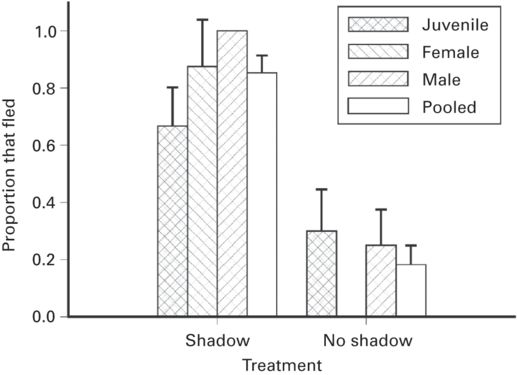
Figure 5.13
The proportion of individuals that flee in Sceloporus virgatus is higher when a shadow suddenly passes over them than when it falls nearby.
(Cooper 2009a)
5.2.1.3.8 Predation pressure
Prey in populations exposed to higher predation pressure may become warier through experience and via natural selection. In studies of seven species in three families, FID was longer for populations having higher predation pressure in six species. In the seventh species, FID was longer where predation pressure was higher in a study limited to two populations of Podarcis lilfordi (Cooper et al. 2009c), but was unrelated to FID in a study of seven populations (Cooper & Pérez-Mellado 2012). For the six species in which FID increased as predation pressure increased, the effect size of predation pressure was large (r = 0.67 ± 0.08). Even with the species lacking an effect added, r = 0.58 ± 0.12 (range 0.00-0.86).
In four species, DF was longer where predation pressure was higher (r = 0.61 ± 0.21; Figure 5.14), but DF and predation pressure were unrelated in two species. Overall, the effect size was intermediate (r = 0.41 ± 0.18, range 0.00-0.98). Very large effect sizes (r ≥ 0.96) occurred for two lacertid species in the Balearic Islands that typically escape without entering refuge in some populations. The proportion of individuals that entered refuges were higher where predation pressure was greater in all of three studies (r = 0.47 ± 0.21, range 0.13-0.84), with a single large effect size in a species that relies heavily on refuges.
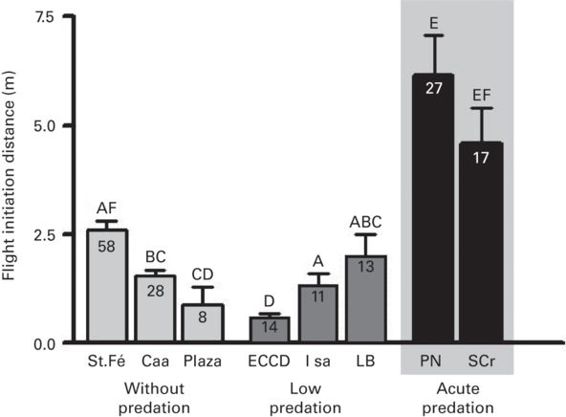
Figure 5.14
In the marine iguana Amblyrhynchus cristatus, FID is longer where predation intensity is greater.
(Berger et al. 2007)
Collectively, the findings confirm the predictions of economic escape theory that FID, DF, and refuge entry increase as risk represented by predation pressure increases. The findings for habituation and predation pressure together suggest that lizard populations adjust FID to frequency of past attacks.
5.2.1.3.9 Predator size and number
Potential prey may be too little to be profitable for predators too large to be overcome (Owen-Smith & Mills 2008). Predation risk increases as the prey’s size brings it into the range favored by the predator. Within the preferred prey size range, the prey’s risk may increase as the size of the predator relative to that of the prey increases. In two lizard species, the proportion of individuals that fled (r = 0.98 ± 0.08, range 0.97-0.99) and FID increased as the size of small predator models increased (Cooper & Stankowich 2010). These findings match predictions based on increase in assessed risk with increase in predator size.
The number of predators approaching simultaneously might also affect FID if the total risk of being captured is greater when more predators approach. In tests at slow approach speed, neither FID nor DF differed for approaches by one predator or two predators side by side; in the same species in using a faster approach speed, FID was longer for approaches by two than one predator, but DF was not affected by number of predators (Cooper et al. 2007). The number of predators was important only at the higher risk level associated with faster approach, but more information is needed on responses of additional prey species.
5.2.1.3.10 Predator facial exposure, direction of gaze, and eye size
Facial exposure of the predator to the prey may indicate that the predator’s field of view includes the prey, and eye contact indicated by directness of gaze is a cue that a predator may have detected the prey. Finally large eye size may indicate high visual acuity and be associated with dangerous predators such as raptors. These factors are expected to increase assessed risk and lengthen FID and DF.
When the predator’s face was exposed to Ctenosaura similis, FID (r = 0.58) and DF (r = 0.52) were longer than when its face was hidden (Burger & Gochfeld 1993). In the same species, FID was longer for direct than averted gaze (r = 0.18), but DF did not differ between direct and averted gaze (Burger et al. 1992). In a different species the proportion of lizards that fled during indirect approaches was much greater as soon as the predator reached a point where the prey passed out of its field of view than at any other point on its approach path (r = 0.98; Cooper 1997b). When escape occurred, the predator’s face was still visible to the prey, but the eyes were not. During indirect approaches, the proportion of lizards that fled, FID, and DF did not differ when the predator looked directly at the lizard or gazed directly ahead along its path (Cooper 2011c). Directness of gaze may be unimportant during indirect approach that would not lead to contact with the prey. Finally, FID (r = 0.37) and DF (r = 0.18) were longer when eye size was artificially increased (Burger et al. 1991).
Lizards use features of the predator’s face and eyes as indicators of the degree of predation risk, but the effects of eye size and directness of gazed were relatively small compared to facial exposure and visibility of eyes. More studies are needed to determine the importance of direct gaze as a predation risk factor.
5.2.2 Cost of fleeing factors that affect FID, DF, and related variables
5.2.2.1 Foraging
Prey in the presence of food must give up a feeding opportunity to flee, which is especially costly when opportunities are limited. Economic escape theory predicts that lizards presented with insects or other food have shorter FID and DF, and that the shortening will be more pronounced as the amount of food present increases. In four species representing four families, FID was shorter when food was present than absent (r = 0.73 ± 0.04, range 0.60-0.81; Figure 5.15). As the number of food items presented to Podarcis lilfordi increased, FID became progressively shorter in (r = 0.93) and DF decreased (r = 0.33; Cooper et al. 2006). Loss of feeding opportunities is a major cost of fleeing. In addition to the above findings, FID and DF were shorter above ground on inflorescences where Podarcis lilfordi licks nectar than on the ground even though lizards above ground had farther to flee (r = 0.40 each; Pérez-Cembranos et al. 2013).
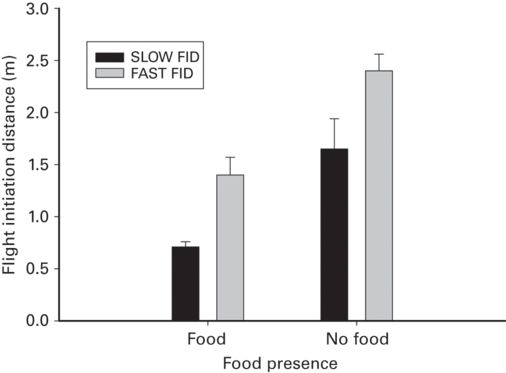
Figure 5.15
Presence of food reduces FID at slow and fast predator approach speeds in Cnemidophorus murinus.
(Cooper et al. 2003)
5.2.2.2 Social costs of fleeing
If a prey may lose a social opportunity by fleeing, its fitness may be impacted through loss of fertilizations or other social benefits. Males have been the focus of almost all studies of social cost of fleeing, but it may affect females too. Male territory holders are predicted to have shorter FID and DF than other males. In the presence of a female, a territorial male is also expected to be less likely than a non-territorial floater male to enter refuge. In species in which males guard females rather than territories, guarding males are predicted to have shorter FID and DF than non-guarding males. If conspecifics are experimentally introduced, males are predicted to have shorter FID because unfamiliar females may be courted and possibly fertilized, whereas introduced males may be rivals.
Territorial males are less likely to enter refuge than are floater males (r = 072; Stapley & Keogh 2004). In another species males have shorter FID when on than off their territories (r = 0.56; Shallenberger 1970). In a third species, Urosaurus ornatus, males having orange-blue throat coloration are territorial, whereas those having the orange throat color morph are non-territorial (Thaker et al. 2009). For this species, FID is shorter for the territorial orange-blue than orange males (r = 0.52; Figure 5.16).
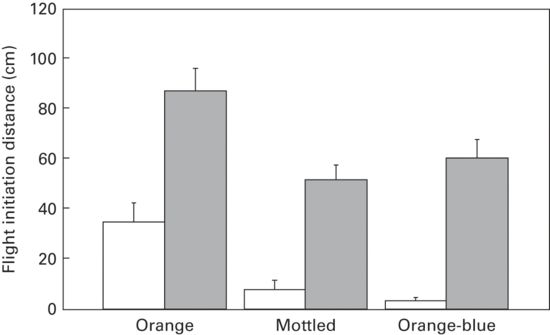
Figure 5.16
In Urosaurus ornatus FID is shorter in territorial males with orange-blue than non-territorial males having orange throat coloration.
(Thaker et al. 2009)
In two species, guarding males had shorter FID than non-guarding males (r = 0.38 ± 0.14, range 0.24-0.52; Figure 5.17), the effect size being larger for a skink than a lacertid. Mate guarding did not affect DF in the sole species studied. In the skink, guarding males had shorter FID than guarded females (r = 0.54; Cooper 1999b), consistent with the greater cost of fleeing for guarding males. In the lacertid being guarded did not affect FID by females (Martín & López 1999).
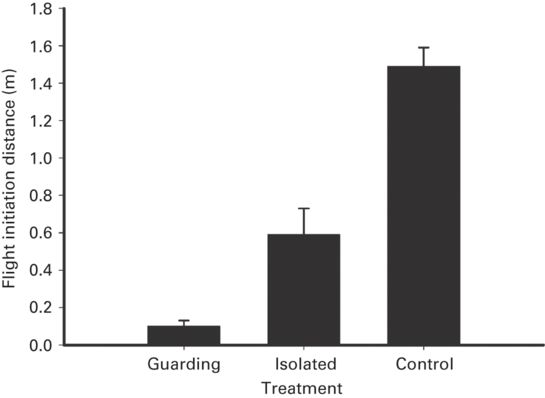
Figure 5.17
Approach distance (FID) in Plestiodon laticeps when a female is introduced is shorter for males guarding females than for solitary males; it is also shorter in solitary males when a female rather than a control stimulus is introduced.
(Cooper 1999b)
When conspecifics are experimentally introduced, FID is consistently affected, but the direction of the effect depends on the sex and circumstances of the focal and introduced lizards. In three species from three families, FID of males was shorter when males rather than control stimuli were introduced (r = 0.57 ± 0.15, range 0.40-0.88). In one of these, the effect of female presence overrode that of approach speed (r = 0.79). Male FID in two species was shorter when females rather than control stimuli were introduced (r = 0.73 ± 0.14, range = 0.60-0.88). In one species, FID of guarding males was longer than that of isolated males when a female was introduced (r = 0.30), which was interpreted as indicating greater potential gain for the isolated male because guarders would have to relax guarding to court an introduced female.
The FID of females in a territorial phrynosomatid species, Sceloporus virgatus, is shorter when a conspecific female rather than a control is introduced (r = 0.72, Cooper 2009b). Such females aggressively reject courtship by males. Failure to do so may be costly, resulting in shorter FID. In a study that did not identify sexes of Phrynosoma modestum, FID was shorter in the presence of a conspecific than when the focal lizard was alone (r = 0.62; Cooper & Sherbrooke 2010).
Collectively, the findings for social costs show that presence of conspecific males or females may impose large costs of fleeing on territorial and mate-guarding males. These costs are reflected in shortened FID as predicted.
5.2.3 Importance of costs of not fleeing and costs of fleeing: relative effect sizes
From the foregoing, it is apparent that FID by lizards is affected by many factors that influence the cost of not fleeing and the cost of fleeing. Effect sizes are highly variable within and between factors. Within a single factor, variation may occur due to interspecific differences or differences in experimental procedures that affect assessed risk and cost of fleeing. Between-factor variation in effect size may indicate which factors are more important, but caution is needed in such interpretations because effects of all but one or two factors are typically held constant in experiments and factors may interact.
With these provisos in mind, some factors clearly have large effects on FID. Here I mention these factors that have been found to have consistent effects in at least four studies. Among predation risk factors, predator traits appear to have the strongest effects, especially approach speed and repeated approach. Habitat factors and prey traits also have fairly strong effects on FID, especially partial concealment of the prey. Distance from refuge, perch height, body temperature, habituation, the prey’s body size, tail loss, conspicuousness, and directness of approach all have substantial effect sizes of 0.40 to 0.56. Foraging and social costs of fleeing also have large effect sizes. These findings indicate that many factors affect escape decisions by lizards. Because effect sizes are affected by levels of other cost of not fleeing and cost of fleeing factors, the effect sizes given cannot be used to rank the importance of the factors. Many factors appear to strongly affect FID, but their relative importance may vary with other risk and cost-of-fleeing factors, as well as with the prey’s fitness when the encounter begins.
5.2.4 Escape strategy
Brief allusions to changes in escape strategy have been made while interpreting effects of predation risk factors. A substantial literature documents differences in escape strategy, but this material is too extensive to be covered here. Selected topics are presented (see Chapter 8).
One way prey can decrease the likelihood of being captured is to decrease the predictability of their escape behavior. Five species of Aspidoscelis fled to diverse microhabitats, exhibited differences in length and linearity of escape runs, and varied in refuge use (Schall & Pianka 1980). The authors argued that escape diversity should increase among conspecific populations as predation pressure increases and should diverge among similar sympatric species having the same predators, but these ideas remain untested.
Locomotor capacities of lizards are related to habitat and escape strategies. Lacertid species occupying more open habitats are faster sprinters having lower endurance and shorter FID than species occupying more vegetated sites; species that use vertical structures tend to be faster climbers (Schulte et al. 2004). Eight scincid species differed in diversity of escape behavior, which was related to differences in microhabitats and locomotor specializations (Melville & Swain 2003). Four species had specialized locomotor abilities and escape behaviors; two species that use a range of microhabitats used more diverse escape behaviors requiring different locomotor skills such as sprinting, jumping, and climbing (Melville & Swain 2003).
Anolis lizards often escape by running along branches or trunks. As branch diameter decreases, running speed decreases and lizards may switch from running to jumping (Losos & Irschick 1996). Because running speed decreases as the angle at branch points increases, anoles prefer escape paths with small angles at branch points (Mattingly & Jayne 2005).
Destinations and types of escape behavior often vary with circumstances. In Uta stansburiana individuals near a cliff fled to it and escaped downward; those located more than 15 m from the cliff fled in circles or non-directionally, perhaps to avoid leaving familiar ground or intruding on territories of conspecifics (Zani et al. 2009). Liolaemus multimaculatus escape by fleeing into patches of grass or burying themselves rapidly in sand. Burying frequency increased as distance to a patch of grass increased (Kacoliris et al. 2009). Differences in escape behavior related to location, habitat structure, and environmental conditions are presumably widespread.
Some lizards undergo ontogenetic color changes and associated changes in escape behavior. Many lizards have brightly colored tails as juveniles that deflect attacks of predators away from the body, permitting escape by caudal autotomy (Cooper & Vitt 1985, 1991). Bright tail coloration increases detectability, but increases escape ability. As lizards grow, they may be less subject to attack by small predators, but more subject to attack by larger, more efficient predators, accounting for ontogenetic loss of bright tail coloration. Another hypothesis, not mutually exclusive, is that hatchlings with brightly colored tails are more active and occupy sites more exposed to predation than adults (Hawlena et al. 2006). In Acanthodactylus beershebensis hatchlings with bright tails were more active in more open locations than older lizards lacking bright tail coloration; they performed deflective tail displays not used by older lizards (Hawlena et al. 2006). Deflective tail displays are much more frequent in juvenile skinks, Plestiodon laticeps, having blue tails than in adults having tan tails (Cooper 1998b).
5.2.5 Latency to flee
When a predator is immobile near a prey that is immobile and aware of the predator, latency to flee is predicted to decrease as cost of not fleeing increases and latency to move is predicted to decrease as opportunity cost of remaining immobile increases (Cooper et al. 2012; Chapter 2). These predictions have been uniformly verified in three studies. Latency to flee was shorter for high risk represented by prior chasing of the prey than for low risk in which the predator had not previously approached (r = 0.50). Latency to flee is shorter when the predator’s approach speed before stopping is faster (r = 0.70 ± 0.04, n = 3); when the predator approaches less directly (r = 0.64 ± 0.03, n = 3); and for the second of two successive approaches (r = 0.50 ± 0.02, n = 2); when the predator maintains eye contact with the prey rather averts its gaze by 30° (r = 0.35, n = 1); and when the predator stands closer to the prey (r = 0.72 ± 0.06, n = 6; Martín et al. 2009; Cooper et al. 2012; Cooper & Sherbrooke 2013b; Figure 5.18). Latency to move is shorter when food is present than absent (r =0.74 ± 0.12, n = 2) and for males in the presence than absence of females (r = 0.87, n = 1) (Cooper & Sherbrooke 2013b).
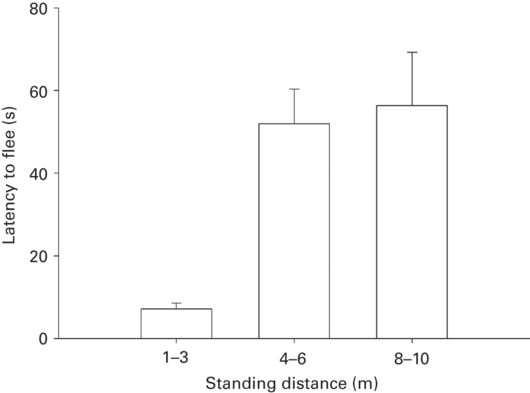
Figure 5.18
Latency to flee in Podarcis lilfordi increases as standing distance increases.
(Cooper et al. 2012)
5.3 Snakes
5.3.1 FID, distance fled, and probability of fleeing
Much less is known about escape decisions by snakes than other lizards. Many snakes permit close approach without moving, relying on crypsis to avoid being detected and/or on envenomation as a defense. Decisions about FID and related variables have been studied for few species (Table 5.1). All but one of these are natricine colubrids, which often occur at high density. For the material below, see Table 5.1 for citations.
Table 5.1 Factors affecting costs of not fleeing (risk) and of fleeing influence escape decisions by snakes.
|
Family/species |
Risk or cost |
Factor |
Metric |
Effect |
Source |
|
Colubridae |
|||||
|
Nerodia sipedon |
risk |
water temperature |
FID |
no effect |
|
|
Nerodia sipedon |
risk |
perch height |
FID |
↑ as PH ↓ |
|
|
Nerodia sipedon |
risk |
starting distance |
FID |
↑ as SD ↑ |
|
|
Nerodia sipedon |
risk |
sex |
FID |
F > M |
|
|
Nerodia sipedon |
risk? |
age |
FID |
no effect |
|
|
Nerodia sipedon |
risk? |
air temperature |
FID |
no effect |
|
|
Nerodia sipedon |
risk |
perch height |
FID |
no effect |
|
|
Regina septemvittata |
risk |
body temperature |
FID |
↑ as BT ↑ |
|
|
Regina septemvittata |
risk? |
sex |
FID |
no effect |
|
|
Regina septemvittata |
risk? |
body length |
FID |
no effect |
|
|
Thamnophis sirtalis |
risk (cost?) |
den vs dispersing |
FID |
dispersing > den |
|
|
Tropidonophis mairii |
risk |
pregnancy |
FID |
↑ |
|
|
Tropidonophis mairii |
cost |
male breeding state |
FID |
NR ↑ R |
|
|
Tropidonophis mairii |
risk? |
age |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
sex |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
body length |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
season |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
air temperature |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
previous capture |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
relative humidity |
FID |
no effect |
|
|
Tropidonophis mairii |
risk? |
moonlight |
FID |
no effect |
|
|
Elapidae |
|||||
|
Pseudonaja textilis |
risk? |
approach speed |
FID |
no effect |
|
|
Pseudonaja textilis |
risk |
snake motion |
FID |
moving > still |
|
|
Pseudonaja textilis |
risk |
adult vs. juvenile |
PF |
J > A |
|
|
Pseudonaja textilis |
risk? |
sex |
PF |
no effect |
|
|
Pseudonaja textilis |
risk? |
wind speed |
FID |
no effect |
|
|
Pseudonaja textilis |
risk |
cover |
FID |
sparse > dense |
|
|
Pseudonaja textilis |
risk |
cover |
PF |
sparse > dense |
|
|
Pseudonaja textilis |
risk |
time of day |
PF |
1001-1800 h > other |
?: tested, but not shown to have an effect; A: adult; BT: body temperature; F: female; FID: flight initiation distance; J: juvenile; NR: non-reproductive; PF: probability of fleeing = proportion of individuals that fled; M: male; PH: perch height; R: reproductive; SD: starting distance.
1: Weatherhead & Robertson 1992; 2: Cooper et al. 2008; 3: Layne & Ford 1984; 4: Shine et al. 2003a; 5: Brown & Shine 2004; 6: Whitaker & Shine: 1999.
Few predation risk factors are known to influence escape decisions by snakes (Table 5.1). As starting distance increased, FID increased. The likelihood of fleeing was greater for snakes in sparser than denser cover, and was longer for moving than immobile snakes (Table 5.1), consistent with predictions of escape theory. Some risk factors have variable effects on FID. No effect was observed for air or water temperature, but FID increased as body temperature increased in Regina septemvitta. Because body temperature affects locomotor capacity, it is more closely related to escape than air or substrate temperature. In one study of Nerodia sipedon, FID decreased as perch height increased, as in lizards, but in another study PH of basking individuals did not affect FID. Differences in distance from shore and availability of refuges might explain differences in the effect of PH.
Sex differences in FID and probability of fleeing were observed in one of four species. Female N. sipedon had longer FID than males, perhaps due to differences in body size or pregnancy. Age and body length did not affect FID in three natricines, but probability of fleeing was greater in juvenile than adult Pseudonaja textilis, which often defend themselves aggressively. One reason for lack of effect of age in natricines may be that hatchlings and juveniles were absent or scarce in the samples.
Several differences in escape between snakes and lizards are known. A finding unique to snakes is that FID is longer for Thamnophis sirtalis while dispersing from hibernacula than at the dens despite similar body size and temperature (Shine et al. 2003a). Several factors might contribute to this result. While dispersing snakes are more likely to be moving and alone. Any risk dilution provided by conspecifics at densely populated dens is lost during dispersal. Because reproduction occurs at dens, snakes there may exhibit shorter FID due to greater cost of fleeing.
Being gravid or pregnant increases body mass and reduces locomotor speed of squamate reptiles (Shine 1980; Cooper et al. 1990). Lizards exhibit shorter FID when gravid because gravid females may change their escape strategies, relying more or crypsis to avoid being detected or staying closer to refuges (section 5.2.1.5). In the natricine T. mairii, FID is longer in pregnant than non-pregnant females, matching the prediction of escape theory for prey that are slowed, but do not alter escape strategy, when pregnant.
That male T. mairii have shorter FID when in reproductive condition than not (Brown & Shine 2004) is also unique among squamates. In lizards, breeding males in the presence of unfamiliar females or guarding mates have shorter FID because fleeing may cost them fertilizations. Although male T. mairii were not guarding mates, fleeing might have reduced the probability of mating with undetected females nearby. However, it is not certain that the effect of reproductive condition is mediated by mating opportunity. Other possible differences in the ecology of breeding and non-breeding males, such as frequency of feeding and associated cost of fleeing or differences in movement or microhabitat use that might affect predation risk, might account for the observed difference.
In the elapid Pseudonaja textilis, approach speed did not affect FID. This lack of relationship does not contradict the prediction of escape theory because P. textilis is a dangerously venomous species that defends itself aggressively when it is likely to be overtaken.
Another way in which escape decisions by snakes may differ from those typical for lizards is that previous capture did not affect FID in Tropidonophis mairii. However, this difference may be a consequence of differences in methods of study. In studies of lizards, initial and subsequent captures occurred within a few days, but T. mairii were collected in a mark-recapture study and the mean intercapture interval was 229 days, obscuring any effect of being captured recently.
Possible effects of several environmental factors that have no readily predictable effect on escape by snakes have been investigated. No effects were shown for time of day, season, relative humidity, moonlight, or wind speed in one species each (see table in the electronic supplementary material). However, season and time of day might have effects mediated by temperature, vegetative cover, and reproductive condition.
Intraspecific variation in FID was pronounced among populations of T. sirtalis from four dens located within 20 m of each other. It is unclear whether the differences reflect genetic variation, differences in exposure to predation, or other factors. In the only comparative study of snakes, FID was longer in N. sipedon than in Thamnopis sauritus; it was intermediate in T. sirtalis, but did not differ in the other two species (Scribner & Weatherhead 1995). Distance fled was longer in N. sipedon than in the other two species (Scribner & Weatherhead 1995).
5.3.2 Refuge selection and entry
Many species of snakes flee to refuges. They may hide in holes, crevices, or under objects, flee into and dive under water, or climb steep slope or plants, or jump from high in trees or on cliffs to prevent predators from pursuing them. However, refuge selection when pursued has received much more anecdotal attention than systematic study. Interspecific differences occur even among closely related species. In the comparative study of natricine snakes (Scribner & Weatherhead 1995), T. sirtalis and T. sauritus often fled into dense vegetation, whereas N. sipedon often dived and swam underwater. These differences in refuge selection reflect differences in habitats because N. sipedon occurred almost exclusively in aquatic habitats, often offshore, whereas the two species of Thamnophis occurred along the shore or inland.
5.3.3 Escape strategy
Many venomous snakes do not flee while being approached, relying instead on deterrence by or use of their chemical defenses. For example, the viper Gloydius shedaoensis, the elapid Psuedonaja textilis, and the laticaudine seasnake (Laticauda colubrina) often do not flee even when harassed (Shine et al. 2002, 2003c). Similarly, North American crotaline vipers often do not flee when approached (my personal observations for several species of Crotalus, Agkistrodon piscivorus, and A. contortrix). With the exception of very large constrictors and cryptically colored species, other snakes lacking such defenses are more likely to flee from predators (Fitch 1963, 1965), especially species capable of very rapid crawling, such as whipsnakes (Masticophis) and racers (Coluber; Fitch 1965).
Color patterns of snakes vary greatly and are related to types of defenses, which were divided into tendency to escape versus defenses other than fleeing (Jackson et al. 1976). In a survey of North American snakes north of Mexico, species having longitudinal striped color patterns and uniform or speckled patterns had higher escape speed and are exposed to visual predators longer each day than snakes having regular cross-banded patterns (perpendicular to the longitudinal axis), irregular banding patterns (varying in shape along the length of the body and not always perpendicular to the longitudinal axis), or blotched-spotted patterns (Jackson et al. 1976).
Snakes having irregular banding and blotched-spotted color patterns tend to have effective defenses other than fleeing, such as envenomation, feigning death, and bluffing. Snakes that are unicolored or unicolored with speckles have a greater tendency to flee and greater flight capability than snakes having irregular banding or blotched-spotted patterns (Jackson et al. 1976). Snakes having striped body patterns exhibit similar traits to those of unicolored or unicolored-speckled snakes, but striped snakes rely to an even greater extent on escape rather than alternative defenses (Jackson et al. 1976). In the northern garter snake, Thamnophis ordinoides, some snakes are striped and others in the same population are not. When neonates were pursued, color morphs with less striping more frequently performed reversals - evasive maneuvers in which the snake suddenly reverses its direction and becomes immobile (Brodie 1989, 1992). Reversal is believed to provide snakes a chance to re-establish crypsis, but striped snakes that employ reversals lose the advantage of difficulty for predators in following movement conferred by striping (Brodie 1989, 1992).
Regularly banded snakes are intermediate in reliance on escape and escape speed to those having the other color patterns; they tend to escape by submerging and to be active in low illumination (Jackson et al. 1976). Jackson et al. (1976) discussed hypotheses suggesting that uniform, striped, and regularly banded patterns mislead visually oriented predators about immobility versus movement and speed, and presented circumstantial evidence that regularly banded patterns may appear to be uniform at speeds above the flicker fusion frequency of a predator (Pough 1976). Regularly banded snakes moving fast enough to surpass the critical flicker fusion frequency would gain an advantage of uniform body coloration, making it difficult for a predator to judge its speed.
Because the Jackson et al. (1976) study was conducted before currently used statistical methods were developed that take phylogenetic relationships into account, it is possible that correlations of traits within genera and families might have produced inflated estimates of significance. The hypothesized mechanisms by which the color patterns create illusions about a fleeing snake’s motion and speed deserve experimental testing.
Snakes that flee in some circumstances may rely on alternate defenses in others. The strategy used varies with temperature, degree of cover, and is likely to vary with other factors that affect escape ability and detectability. Temperature affects probability of fleeing via its effect on locomotor capacity, and other variables. The shorter FID at lower body temperatures in R. septemvittata hints that snakes may switch from escape to other defenses when body temperature falls too low for rapid crawling. In the natricine Thamnopis sirtalis (Shine et al. 2003b) and the African viper Bitis schneideri (Maritz 2012), the probability of fleeing is reduced at lower temperatures. Because the data for B. schneideri were combined, approach and a 30-s interval during which the snakes’ heads were touched five times, it is uncertain whether FID or probability of fleeing were affected by approach alone. The probability of fleeing by P. textilis is lower in sparser than denser cover (Whittaker and Shine, 1999). It may be predicted that probability of fleeing is reduced at low levels of illumination.
Ecological differences between species may account for differences in escape behavior in two elapids. Cryptophis nigrescens hunts actively in open areas at night, whereas Hoplocephalus bungaroides ambush their prey in retreat sites in sun-warmed rocks (Llewelyn et al. 2010). Both species relied primarily on fleeing to escape, but the antipredatory behavior of C. nigrescens, which is exposed to cool temperatures while foraging in the open, was less sensitive to thermal variation and its use of retaliatory defense was less frequent than that of H. bungaroiedes (Llewelyn et al. 2010).
5.4 Crocodilians, sphenodontidans, and turtles
Literature searches did not reveal any papers about FID (or its synonyms) for crocodilians, tuataras, or turtles. Alligators and other crocodilians often escape when approached by diving beneath the water surface. Tuataras escape into burrows. Aquatic and semi-aquatic turtles typically escape by diving; such turtles often flee to water if encountered near shore or drop off logs or bushes into water when approached. Many turtles use their shells as refuges when overtaken on land or captured in water. Terrestrial turtles and some semi-aquatic species have shells that permit complete or nearly complete withdrawal of vulnerable body parts and, in some the shells close, effectively blocking access by predators to soft body parts.
As discussed in Chapter 9, when the turtle Mauremys leprosa is approached while on land, it attempts to flee to water, but if overtaken withdraws into its shell (Martín et al. 2003). In terrestrial turtles, running speed is too slow for escape from typical predators. When a predator draws near, these turtles escape not by fleeing, but by withdrawing their hands and necks, legs, and often tails inside their shells. Aquatic turtles and semi-aquatic turtles in water are more likely than terrestrial turtles to flee.
5.5 Future directions
It is apparent from the foregoing that research on snakes, turtles, tuataras, and crocodilians is needed. Incorporating effects of aspects of reptilian cognition, such as learning local escape routes and refuge sites, in escape studies would also be valuable. Studies of escape by reptiles have focused on effects of costs of not fleeing and costs of fleeing in single species. Only recently have studies been published using the comparative method. As data for more species accumulate, comparative studies should make substantial contributions to our knowledge of topics such as relationships of escape decisions to locomotor capacity, sensory ability, morphological traits, ecology, and phylogeny.
References
Bateman, P. W. & Fleming, P. A. (2009). To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. Journal of Zoology, 277, 1-14.
Berger, S., Wikelski, M., Romero, L. M., Kalko, E. K. V. & Rödl, T. (2007). Behavioral and physiological adjustments to new predators in an endemic island species, the Galápagos marine iguana. Hormones and Behavior, 52, 653-663.
Bisazza, A., Rogers, L. J. & Vallortigarac, G. (1998). The origins of cerebral asymmetry: A review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neuroscience and Biobehavioral Reviews, 22, 411-426.
Blamires, S. J. (1999). Factors influencing the escape response of an arboreal agamid lizard of tropical Australia (Lophognathus temporalis) in an urban environment. Canadian Journal of Zoology, 77, 1998-2003.
Blumstein, D. T. (2003). Flight-initiation distance in birds is dependent on intruder starting distance. Journal of Wildlife Management, 67, 852-857.
Brodie, E. D., III. (1989). Genetic correlations between morphology and antipredator behavior in natural populations of the garter snake Thamnophis ordinoides. Nature, 342, 542-543.
Brodie, E. D., III (1992). Correlational selection for clolor patterns and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution, 46, 1284-1298.
Brown, G. P. & Shine, R. (2004). Effects of reproduction on the antipredator tactics of snakes (Tropidophis mairii, Colubridae). Behavioral Ecology and Sociobiology, 56, 257-262.
Bulova, S. J. (1994). Ecological correlates of population and individual variation in antipredator behavior of two species of desert lizards. Copeia, 1994, 980-992.
Burger, J. & Gochfeld, M. (1993). The importance of the human face in risk perception by black iguanas, Ctenosaura similis. Journal of Herpetology, 27, 426-430.
Burger, J., Gochfeld, M. & Murray, B. G., Jr. (1991). The role of a predator’s eye size in risk perception by basking black iguana, Ctenosaura similis. Animal Behaviour, 42, 471-476.
Burger, J., Gochfeld, M. & Murray, B. B., Jr. (1992). Risk discrimination of eye contact and directness of approach in black iguanas (Ctenosaura similis). Journal of Comparative Psychology, 106, 97-101.
Clark, C. W. (1994). Antipredator behavior and the asset-protection principle. Behavioral Ecology, 5, 159-170.
Congdon, J. D., Vitt, L. J. & King, W. W. (1974). Geckos: Adaptive significance and energetics of tail autotomy. Science, 184, 1379-1380.
Cooper, W. E., Jr. (1997a). Escape by a refuging prey, the broad-headed skink (Eumeces laticeps). Canadian Journal of Zoology, 75, 943-947.
Cooper, W. E., Jr. (1997b). Threat factors affecting antipredatory behavior in the broad-headed skink (Eumeces laticeps): Repeated approach, change in predator path, and predator’s field of view. Copeia, 1997, 613-619.
Cooper, W. E., Jr. (1998a). Effects of refuge and conspicuousness on escape behavior by the broad-headed skink (Eumeces laticeps). Amphibia-Reptilia, 19, 103-108.
Cooper, W. E., Jr. (1998b). Reactive and anticipatory display to deflect predatory attack to an autotomous lizard tail. Canandian Journal of Zoology, 76, 1507-1510.
Cooper, W. E., Jr. (1998c). Direction of predator turning, a neglected cue to predation risk. Behaviour, 135, 55-64.
Cooper, W. E., Jr. (1999a). Escape behavior by prey blocked from entering the nearest refuge. Canadian Journal of Zoology, 77, 671-674.
Cooper, W. E., Jr. (1999b). Tradeoffs between courtship, fighting, and antipredatory behavior by a lizard, Eumeces laticeps. Behavioral Ecology and Sociobiology, 47, 54-59.
Cooper, W. E., Jr. (2003). Shifted balance of risk and cost after autotomy affects use of cover, escape, activity, and foraging in the keeled earless lizard (Holbrookia propinqua). Behavioral Ecology and Sociobiology, 54, 179-187.
Cooper, W. E., Jr. (2006a). Dynamic risk assessment: Prey rapidly adjust flight initiation distance to changes in predator approach speed. Ethology, 112, 858-864.
Cooper, W. E., Jr. (2006b). Risk factors affecting escape grahamÿr by Puerto rican Anolis lizards. Canadian Journal of Zoology, 84, 495-504.
Cooper, W. E., Jr. (2007a). Escape and its relationship to pursuit-deterrent grahamÿrÿ in the Cuban curly-tailed lizard Leiocephalus carinatus. Herpetologica, 63, 144-150.
Cooper, W. E., Jr. (2007b). Compensatory changes in escape and refuge use following autotomy in the lizard Sceloporus virgatus. Canadian Journal of Zoology, 85, 99-107.
Cooper, W. E., Jr. (2007c). Foraging modes as suites of coadapted movement traits. Journal of Zoology, 272, 45-56.
Cooper, W. E., Jr. (2008). Strong artifactual effect of starting distance on flight initiation distance in the actively foraging lizard Aspidoscelis exsanguis. Herpetologica, 64, 200-206.
Cooper, W. E., Jr. (2009a). Rapid covering by shadow as a cue to predation risk in three lizard species. Behaviour, 146, 1217-1234.
Cooper, W. E., Jr. (2009b). Flight initiation distance decreases during social activity in lizards (Sceloporus virgatus). Behavioral Ecology and Sociobiology, 63, 1765-1771.
Cooper, W. E., Jr. (2009c). Fleeing and hiding under simultaneous risks and costs. Behavioral Ecology, 20, 665-671.
Cooper, W. E., Jr. (2010a). Pursuit deterrence varies with predation risks affecting escape behavior in the lizard Callisaurus draconoides. Animal Behaviour, 80, 249-256.
Cooper, W. E., Jr. (2010b). Escape tactics and effects of perch height and habituation on flight initiation distance in two Jamaican anoles (Squamata: Polychrotidae). Revista de Biologia Tropical, 58, 1199-1209.
Cooper, W. E., Jr. (2011a). Age, sex and escape grahamÿr in the striped plateau lizad (Sceloporus virgatus) and the mountain spiny lizard (Sceloporus jarrovii), with a review of age and sex effects on escape by lizards. Behaviour, 148, 1215-1238.
Cooper, W. E., Jr. (2011b). Pursuit deterrence, predations risk, and escape in the lizard Callisaurus draconoides. Behavioral Ecology and Sociobiology, 65, 1833-1841.
Cooper, W. E., Jr. (2011c). Influence of some potential predation risk factors and interaction between predation risk and cost of fleeing on escape by the lizard Sceloporus virgatus. Ethology, 117, 620-629.
Cooper, W. E., Jr. (2012). Risk factors affecting escape behavior by the Jamaican lizard Anolis lineatopus (Polychrotidae, Squamata). Caribbean Journal of Science, 46, 1-12.
Cooper, W. E., Jr. & Avalos, A. (2010). Predation risk, escape and refuge use by mountain spiny lizards (Sceloporus jarrovii). Amphibia-Reptilia, 31, 363-373.
Cooper, W. E., Jr. & Blumstein, D. T. (2014). Starting distance, alert distance and flushing early challenge economic escape theory: New proposed effects on costs of fleeing and not fleeing. Behavioral Ecology, 25, 44-52.
Cooper, W. E., Jr. & Frederick, W. G. (2007). Optimal flight initiation distance. Journal of Theoretical Biology, 244, 59-67.
Cooper, W. E., Jr. & Pérez-Mellado, V. (2011). Escape by the Balearic lizard (Podarcis lilfordi) is affected by elevation of an approaching predator, but not by some other potential predation risk factors. Acta Herpetologica, 6, 247-259.
Cooper, W. E., Jr. & Pérez-Mellado, V. (2012). Historical influence of predation pressure on escape behavior by Podarcis lizards in the Balearic islands. Biological Journal of the Linnaean Society, 107, 254-268
Cooper, W. E., Jr. & Sherbrooke, W. C. (2010). Crypsis influences escape decisions in the round-tailed horned lizard (Phrynosoma modestusm). Canadian Journal of Zoology, 88, 1003-1010.
Cooper, W. E., Jr. & Sherbrooke, W. C. (2012). Choosing between a rock and a hard place: Camouflage in the round-tailed horned lizard Phrynosoma modestum. Current Zoology, 58, 541-548.
Cooper, W. E., Jr. & Sherbrooke, W. C. (2013a). Effects of recent movement, starting distance and other risk factors on escape behaviour by two phrynosomatid lizards. Behaviour, 150, 447-469.
Cooper, W. E., Jr. & Sherbrooke, W. C. (2013b). Risk and cost of immobility in the presence of an immobile predator: effects on latency to flee or approach food or a potential mate. Behavioral Ecology and Sociobiology, 67, 583-592.
Cooper, W. E. & Stankowich, T. (2010). Prey or predator? Body size of an approaching animal affects decisions to attack or escape. Behavioral Ecology, 21, 1278-1284.
Cooper, W. E., Jr. & Vitt, L. J. (1985). Blue tails and autotomy: Enhancement of predation avoidance in juvenile skinks. Zeitschrift fur Tierpsychologie, 70, 265-276.
Cooper, W. E., Jr. & Vitt, L. J. (1991). Influence of detectability and ability to escape on natural selection of conspicuous autotomous defenses. Canadian Journal of Zoology, 69, 757-764.
Cooper, W. E., Jr. & Wilson, D. S. (2007). Beyond optimal escape theory: Microhabitats as well as predation risk affect escape and refuge use by the phrynosomatid lizard Sceloporus virgatus. Behaviour, 144, 1235-1254.
Cooper, W. E., Jr. & Wilson, D. S. (2008). How to stay alive after losing your tail. Behaviour, 145, 1085-1089.
Cooper, W. E., Jr., Vitt, L. J., Hedges, R. & Huey, R. B. (1990). Locomotor impairment and defense in gravid lizards (Eumeces laticeps): Behavioral shift in activity may offset costs of reproduction in an active forager. Behavioral Ecology and Sociobiology, 27, 153-157.
Cooper, W. E., Jr., Pérez-Mellado, V., Baird, T. et al. (2003). Effects of risk, cost, and their interaction on optimal escape by nonrefuging Bonaire whiptail lizards, Cnemidophorus murinus. Behavioral Ecology, 14, 288-293.
Cooper, W. E., Jr., Perez-Mellado, V. & Hawlena, D. (2006). Magnitude of food reward affects escape behavior and acceptable risk in Balearic lizards, Podarcis lilfordi. Behavioral Ecology, 17, 554-559.
Cooper, W. E., Jr., Perez-Mellado, V. & Hawlena, D. (2007). Number, speeds, and approach paths of predators affect escape behavior by the Balearic lizard, Podarcis lilfordi. Journal of Herpetology, 41, 197-204.
Cooper, W. E., Jr., Attum, O. & Kingsbury, B. (2008). Escape behaviors and flight initiation distance in the common water snake Nerodia sipedon. Journal of Herpetology, 42, 493-500.
Cooper, W. E., Jr., Wilson, D. S. & Smith, G. R. (2009a). Sex, reproductive status, and cost of tail autotomy via decreased running speed. Ethology, 115, 7-13.
Cooper, W. E., Jr., Hawlena, D. & Pérez-Mellado, V. (2009b). Interactive effect of starting distance and approach speed on escape challenges theory. Behavioral Ecology, 20, 542-546.
Cooper, W. E., Jr., Hawlena, D. & Pérez-Mellado, V. (2009c). Islet tameness: Escape behavior and refuge use in populations of the Balearic lizard (Podarcis lilfordi) exposed to differing predation pressure. Canadian Journal of Zoology, 87, 912-919.
Cooper, W. E., Jr., López, P., Martín, J. & Pérez-Mellado, V. (2012). Latency to flee from an immobile predator: Effects of risk and cost of immobility for the prey. Behavioral Ecology, 23, 790-797.
Eifler, D. (2001). Egernia cunninghami (Cunningham’s skink). Escape behavior. Herpetological Review, 32, 40.
Fitch, H. S. (1963). Natural history of the racer Coluber constrictor. University of Kansas Publications, Museum of Natural History, 15, 351-468.
Fitch, H. S. (1965). An ecological study of the garter snake, Thamnophis sirtalis. University of Kansas Publications, Museum of Natural History, 15, 493-564.
Hawlena, D., Boochnik, R., Abramsky, Z. & Bouskila, A. (2006). Blue tail and striped body: Why do lizards change their infant costume when growing up? Behavioral Ecology, 17, 889-896.
Hawlena, D., Perez-Mellado, V. & Cooper, W. E., Jr. (2009). Morphological traits affect escape behavior of the Balearic lizards (Podarcis lilfordi). Amphibia-Reptilia, 30, 587-592.
Hertz, P. E., Huey, R. B. & Nevo, E. (1983). Homage to Santa Anita: Thermal sensitivity of sprint speed in agamid lizards. Evolution, 37, 1075-1084.
Holley, A. J. F. (1993). Do brown hares signal foxes? Ethology, 94, 21-30.
Huey, R. B. (1982). Temperature, physiology, and the ecology of reptiles. In Biology of the Reptilia, Vol. 12, Physiology C: Physiological Ecology. London: Academic Press, pp. 25-91.
Jackson, J. F., Ingram, W., III. & Campbell, H. W. (1976). The dorsal pigmentation as an antipredator strategy: a multivariate approach. American Naturalist, 110, 1029-1053.
Kacoliris, F. P., Gurrero, E., Molinari, A., Moyano, B. & Rafael, A. (2009). Run to shelter or bury into the sand? Factors affecting escape grahamÿr decisions in Argentinian sand dun lizards (Liolaemus multimaculatus). Herpetological Journal, 19, 213-216.
Kelt, D. A., Nabors, L. K. & Forister, M. L. (2002). Size-specific differences in tail loss and escape behavior in Liolaemus nigromaculatus. Journal of Herpetology, 36, 322-325.
Kramer, D. L. & Bonenfant, M. (1997). Direction of predator approach and the decision to flee to a refuge. Animal Behaviour, 54, 289-295.
Lailvaux, S. P., Alexander, G. J. & Whiting, M. J. (2003). Sex-based differences and similarities in locomotor performance, thermal preferences, and escape behaviour in the lizard Platysaurus intermedius. Physiological and Biochemical Zoology, 76, 511-521.
Layne, J. R. & Ford, N. B. (1984). Flight distance of the queen snake, Regina septemvittata. Journal of Herpetology, 18, 496-498.
Lima, S. L. & Bednekoff, P. A. (1999). Temporal variation in danger drives antipredator American Naturalist, 153, 649-659.
Llewelyn, J., Webb, J. K. & Shine, R. (2010). Flexible defense: context-dependent antipredator responses of two species of Australian elapid snakes. Herpetologica, 66, 1-11.
López, P., Hawlena, D., Polo, V., Amo, L. & Martín, J. (2005). Sources of shy-bold variations in antipredator grahamÿr of male Iberian rock lizards. Animal Behaviour, 69, 1-9.
Losos, J. B. & Irschick, D. J. (1996). The effect of perch diameter on escape grahamÿr of Anolis lizards: Laboratory predictions and field tests. Animal Behaviour, 51, 593-602.
Losos, J. B., Mouton, P. L. F. N., Bickel, R., Cornelius, I. & Ruddock, L. (2002). The effect of body armature on escape behaviour in cordylid lizards. Animal Behaviour, 64, 313-321.
Maritz, B. (2012). To run or hide? Escape behavior in a cryptic African snake. African Zoology, 47, 270-274.
Martín, J. & López, P. (1999). Nuptial coloration and mate-guarding affect escape decisions of male lizards, Psammodromus algirus. Ethology, 105, 439-447.
Martín, J., López, P. & Cooper, W. E., Jr. (2003). When to come out from a refuge: balancing predation risk and foraging opportunities in an alpine lizard. Ethology, 109, 77-87.
Martín, J., Luque-Larena, J. J. & López, P. (2009). When to run from an ambush predator: balancing crypsis benefits with costs of fleeing in lizards. Animal Behaviour, 78, 1011-1018.
Mattingly, W. B. & Jayne, B. C. (2005). The choice of arboreal escape paths and its consequences for the locomotor behaviour of four species of Anolis lizards. Animal Behaviour, 70, 1239-1250.
McConnachie, S. & Whiting, M. J. (2003). Costs associated with tail autotomy in an ambush foraging lizard, Cordylus melanotus melanotus. African Zoology, 38, 57-65.
Melville, J. & Swain, R. (2003). Evolutionary correlations between escape behaviour and performance ability in eight species of snow skinks from Tasmania (Niveoscincus: Lygosominae). Journal of Zoology, 261, 79-89.
Olsson, M., Shine, R. & Bak-Olsson, E. (2000). Locomotor impairment of gravid lizards: is the burden physiological? Journal of Evolutionary Biology, 13, 263-268.
Owen-Smith, N. & Mills, M. G. L. (2008). Predator-prey size relationships in an African large-mammal food web. Journal of Animal Ecology, 77, 173-183.
Pérez-Cembranos, A., Pérez-Mellado, V. & Cooper, W. E. (2013). Predation risk and opportunity cost of fleeing while foraging on plants influences escape decisions of and insular lizard. Ethology, 119, 522-530.
Pough, F. H. (1976). Multiple cryptic effects of crossbanded and ringed patterns of snakes. Copeia, 1976, 834-836.
Ruxton, G. D., Sheratt, T. N. & Speed, M. (2004). Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford: Oxford University Press.
Schall, J. J. & Pianka, E. R. (1980). Evolution of escape behavior diversity. American Naturalist, 115, 551-556.
Schneider, K. R., Parmerlee, J. S., Jr. & Powell, R. (2000). Escape behavior of Anolis lizards from the Sierra de Baoruco, Hispaniola. Caribbean Journal of Science, 36, 321-323.
Schulte, J. A., Losos, J., Cruz, F. B. & Nunez, H. (2004). The relarionship between morphology, escape behavior and microhabitat occupation in the lizard clade Liolaemus (Iguanidae: Tropidurinae: Liolaemini). Journal of Evolutionary Biology, 17, 408-420.
Schwarzkopf, L. & Shine, R. (1992). Costs of reproduction in lizards: escape tactics and susceptibility to predation. Behavioral Ecology and Sociobiology, 31, 17-25.
Scribner, S. J. & Weatherhead, P. J. (1995). Locomotion and antipredator behaviour in three species of aquatic snakes. Canadian Journal of Zoology, 73, 321-329.
Shallenberger, E. W. (1970). Tameness in Insular Animals: a Comparison of Approach Distances of Insular and Mainland Iguanid Lizards. Los Angeles: University of California at Los Angeles.
Shine, R. (1980). “Costs” of reproduction in reptiles. Oecologia, 46, 92-100.
Shine, R., Sun, L.-X., Fitzgerald, M. & Kearney, M. (2002). Antipredator responses of free-ranging pit vipers (Gloydius shedaoensis, Viperidae). Copeia, 2002, 843-850.
Shine, R., Phillips, B., Waye, H. & Mason, R. T. (2003a). Behavioral shifts associated with reproduction in garter snakes. Behavioral Ecology, 14, 251-256.
Shine, R., Phillips, B., Waye, H. & Mason, R. T. (2003b). Small-scale geographic variation in antipredator tactics of garter snakes. Herpetologica, 59, 333-339.
Shine, R., Bonnett, X. & Cogger, H. C. (2003c). Antipredator tactics of amphibious sea snakes (Serpentes, Laticaudidae). Ethology, 109, 533-542.
Smith, G. R. (1996). Correlates of approach distance in the striped plateau lizard (Sceloporus virgatus). Herpetological Journal, 6, 56-58.
Stankowich, T. & Blumstein, D. T. (2005). Fear in animals: a meta-analysis and review of risk assessment. Proceedings of the Royal Society of London, Series B, Biological Sciences, 272, 2627-2634.
Stankowich, T. & Coss, R. G. (2007). Effects of risk assessment, predator behavior, and habitat on escape behavior in Columbian black-tailed deer. Behavioral Ecology, 18, 358-367.
Stapley, J. & Keogh, J. S. (2004). Exploratory and antipredator behaviours differ between territorial and nonterritorial male lizards. Animal Behaviour, 68, 841-846.
Stiller, R. B. & McBrayer, L. B. (2013). The ontogeny of escape behavior, locomotor performance, and the hind limb in Sceloporus woodi. Zoology, 116, 175-181.
Thaker, M., Lima, S. L. & Hews, D. K. (2009). Alternative antipredatory tactics in tree lizard morphs: hormonal and behavioural responses to a predator encounter. Animal Behaviour, 77, 395-401.
Ward, J. P. & Hopkins, W. D. (1993). Primate laterality: current behavioral evidence of primate asymmetries. New York: Springer-Verlag.
Weatherhead, P. J. & Robertson, I. C. (1992). Thermal constraints on swimming performance and escape response of northern water snakes (Nerodia sipedon). Canadian Journal of Zoology, 70, 94-98.
Whitaker, P. B. & Shine, R. (1999). Responses of free-ranging brownsnakes (Pseudonaja textilis: Elapidae). Wildlife Research, 26, 689-704.
Williams, E. E. (1983). Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In Lizard Ecology: Studies of a Model Organism. Cambridge: Harvard University Press, pp. 326-370.
Williams, D. M., Samia, D. S. M., Cooper, W. E., Jr. & Blumstein, D. T. (2014). The flush early and avoid the rush hypothesis holds after accounting for spontaneous behavior. Behavioral Ecology, 25, 1136-1147.
Zani, P. A., Jones, T. D., Neuhaus, R. A. & Milgrom, J. E. (2009). Effect of refuge distance on escape behavior of side-blotched lizards (Uta stansburiana). Canadian Journal of Zoology, 87, 407-414.