The Human Side of Science: Edison and Tesla, Watson and Crick, and Other Personal Stories behind Science's Big Ideas (2016)
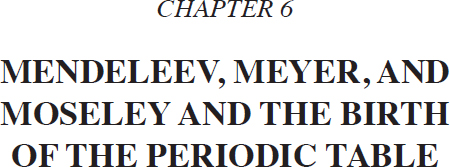
The two most common elements in the universe are hydrogen and stupidity.
—Harlan Ellison, American speculative fiction writer1
Chemists ![]() the periodic table. It is a one-stop shop for element symbols, chemical properties, physical properties, atomic mass, atomic number, valences, and plenty of other valuable information. Its one-hundred-plus elements, all arranged in neat rows and columns, might tend to make you suspicious that many clever people were involved in building the table from Aristotle's notion of elements being fire, air, earth, and water. Right. For that matter, how do elements relate to the existence of atoms, denied vehemently by Aristotle (see chapter 1)?
the periodic table. It is a one-stop shop for element symbols, chemical properties, physical properties, atomic mass, atomic number, valences, and plenty of other valuable information. Its one-hundred-plus elements, all arranged in neat rows and columns, might tend to make you suspicious that many clever people were involved in building the table from Aristotle's notion of elements being fire, air, earth, and water. Right. For that matter, how do elements relate to the existence of atoms, denied vehemently by Aristotle (see chapter 1)?
For a long time, scientists ignored this question. Many suspected there were indeed such things as atoms, but they had only vague ideas about what these atoms might look like. Some scientists avoided the question by dealing only with matter in bulk: Whatever particles cannonballs are made of, a cannonball will still travel a distance of x when propelled by an amount of gunpowder y and shot at an angle z. In other words, these scientists acted like physicists. Other scientists focused on interactions between substances: If substance A is mixed with substance B, stirred vigorously and heated, substance C is formed. These scientists started as alchemists, trying to turn base metals into gold, but they later evolved into chemists.
Robert Boyle (Robert Hooke's mentor; see chapter 3), in 1661, defined elements as “certain primitive and simple, or perfectly unmingled bodies; which are not being made of any other bodies.”2 This was a good start, but it was ignored.
Antoine Lavoisier (see chapter 5) initiated the shift from alchemy to chemistry with his precise measurements using balances. In his book Traité élémentaire de Chimie (The Elementary Treatise on Chemistry), he listed thirty-three simple substances, twenty-three of which we now recognize as elements. Although his lab work yielded some values of the relative masses, his list of simple substances, which included light and caloric, didn't include masses. His definition of an element was the “last point which analysis is capable of reaching”;3 in other words, the point beyond which no additional parts are discovered. He also named oxygen and hydrogen, but he couldn't bring himself to accept the reality of atoms because he thought they were philosophically impossible.
DALTON TO THE RESCUE
An unlikely fellow showed up around 1800 to put atoms on a more solid footing: John Dalton.
John Dalton was born in 1766 to a modest Quaker family of tradesmen. He was educated in a Quaker elementary school, an alternative to English schools. Because Quakers didn't belong to the Church of England, they were regarded as dissenters. Dalton's older brother had taken over the Quaker school in Manchester, England, known as “New College,” and although Dalton was only twelve years old, he assisted his brother by teaching there. He continued teaching until age thirty-four, when the school experienced financial difficulty. He then resigned to become a private tutor. One of his major interests had been meteorology, the study of weather. From his measurements on the properties of air, Dalton supported Democritus's notion of atoms, but he added the idea that atoms differed from each other in size and mass. In a paper read to the Manchester Literary and Philosophical Society in 1803, he said, “An inquiry into the relative weights of the ultimate particles of Bodies is a subject, as far as I know, entirely new.” Further, atoms combine with each other in fixed proportions to form compounds in ratios of small, whole numbers. This is called the law of multiple proportions, which, according to Swiss chemist J. J. Berzelius, would be “a mystery without the atomic theory.”

John Dalton (1766-1844). From Wikimedia Commons, user Materialscientist.

Used with permission from Sidney Harris.
Dalton identified six elements (collections of the same atoms): hydrogen, oxygen, nitrogen, carbon, sulfur, and phosphorus, along with their relative masses, based largely on his and other chemists’ measurements of gases.
As we will see, the modern-day list of elements includes more than one hundred different types, none of which turn out to be earth, water, air, fire, or “æther,” as Aristotle had claimed (see chapter 1).
Nineteenth-century chemists continued their quest to discover new elements and measure the atomic mass of each element as well as its chemical properties. The lowest atomic mass was defined to be one and was assigned to the element hydrogen (H). (Chemists abbreviate the names of the elements with one or two letters.) In the early 1800s the following elements were known, along with their approximate atomic masses:

LOOKING FOR PATTERNS
First in 1817 and then more thoroughly in 1829, German chemist Johann Döbereiner (1780-1849)—a personal friend of the famous German writer Johann Goethe—published articles in which he examined the properties of sets of elements that he called triads (for example, lithium, sodium, and potassium). The elements of each triad have similar chemical properties, and the atomic mass of the second element of the triad is approximately equal to the average of the atomic masses of the other two elements. For example, lithium 7 + potassium 39 = 46. Half of 46 is 23, the mass of sodium. The same relationship worked for two other groups of elements. Döbereiner thought this too strange to be a mere coincidence. He felt that there must be some kind of pattern involved. He proposed that all elements could be grouped in such triads, however subsequent attempts to expand the concept to other groupings were unsuccessful. His attempt failed to provide a comprehensive framework, in part because the masses of elements were measured inaccurately and in nonstandard ways.
Another attempt to organize the elements into a pattern came from an unlikely source: French geologist Alexandre-Émile Béguyer de Chancourtois. In 1862, he arranged the known elements on a cylinder in order of increasing atomic mass and noted that sometimes similar elements having similar chemical properties recurred periodically. Unfortunately, the paper describing his system left out the diagram and included many geological ideas, and so was ignored by chemists of the time.
In the years 1863 to 1866, English chemist John Newlands (1837-1898) proposed another idea: the law of octaves. Newlands stated that when the elements are listed in increasing atomic mass, the eighth element is similar in chemical properties to the first, the ninth to the second, and so on, just like notes in musical octaves. Unfortunately, Newlands's “law of octaves” did not seem to work for elements heavier than calcium, and so his idea was publicly ridiculed. At one scientific meeting, Newlands was asked why he didn't just arrange the elements in alphabetical order instead of by atomic mass, since that would make as much sense as his octaves. Newlands had carried the idea of the metaphor too far; the actual relationship is not so simple. His work was therefore not taken seriously by other chemists.
WILLIAM ODLING'S OFT-FORGOTTEN CONTRIBUTION
In the 1860s, a highly respected academic English chemist, William Odling (1829-1921), devised a table of elements using repeating units of seven elements before starting a new row. Some elements were omitted, however, without any reasonable explanation. Odling considered the scheme to be a simple convenience, and he was not committed to atomism, so the masses of elements didn't play a large role in his scheme. He did not receive recognition on par with other researchers because he didn't advocate for his system strongly enough, so his colleagues regarded it as mere speculation.
THE FIRST INTERNATIONAL CHEMISTRY CONFERENCE
The Karlsruhe Congress of September 1860 brought European chemists together for the first time to discuss many important matters of a chemical nature, including the standardization of atomic masses (also referred to as atomic weights). This meeting, which may have sparked the eventual organization of the International Union of Pure and Applied Chemistry (IUPAC), inspired many to attempt to deal with finding organizational patterns in the ever-increasing number of chemical elements. Among those in attendance were two who had a profound effect on finding those patterns: Julius Lothar Meyer and Dmitri Mendeleev.

Julius Lothar Meyer (1830-1895). From Wikimedia Commons, user Kelson.
Julius Lothar Meyer was born in Varel, Germany, in 1830 to an academically oriented family. His father was a doctor, and his mother's family also included physicians. He studied at Zurich, Würzburg, and Heidelberg, earning a medical degree and a PhD in chemistry along the way. His dissertation dealt with carbon monoxide in the blood, and he noted the combination of oxygen with hemoglobin in the blood.
Soon after attending the Karlsruhe Congress in 1860, Meyer wrote a book, Die modernen Theorien der Chemie (The Modern Theory of Chemistry). At 164 pages, it is more like a long treatise than a textbook, but this 1864 work includes a table of elements that exhibit a periodic repetition of chemical properties as the atomic mass increases. Befitting the slim nature of the publication, the table is short: it contains only twenty-eight elements. Meyer was a proponent of atomism, but he clearly chose not to make any predictions about gaps in the table representing missing elements. As time moved along, Meyer augmented and revised this book and used it as a teaching tool, mostly for graduate students. The next time the table was revised was in 1869, but its publication didn't occur until 1870. The updated periodic table was almost identical to another table that had been published in 1869. So, where did the other table come from; who beat Meyer into print by a few months?
MENDELEEV TO THE RESCUE
Of all the six “periodic” tables introduced in the period between 1860 and 1870 the last one came from the Russian chemist, Dmitri Mendeleev (1834-1907). Dmitri Mendeleev was the last child (of eleven to sixteen; the number is unclear) born to Ivan Pavlovich Mendeleev and Maria Dmitrievna Mendeleeva (née Kornilieva) near Tobolsk in Siberia. His father taught fine art, politics, and philosophy at the local gymnasium, but he developed cataracts and lost his sight and his job. His mother restarted an abandoned glass factory owned by the family and operated it for several years until it burned down. Because Mendeleev demonstrated early scientific talent, his mother set out to take him to Moscow, the home of her wealthy brother, so he could continue his education at a first-class institution. The social turmoil in Moscow (revolts in nearby Romania and Hungary) prevented non-Muscovites from being admitted to the university. Mendeleev, his sister, and his mother continued their search for education in the Russian capital, St. Petersburg, but met the same fate there. Finally, at the Main Pedagogical Institute in St. Petersburg, a professor who knew Mendeleev's father when he attended there recommended Mendeleev, and he was admitted. Mendeleev endured a rocky start as a student at the Institute. His mother and sister contracted and died of tuberculosis, and he, too, became ill. In spite of poor early grades; his illness, which necessitated classmates bringing him books in the hospital; and his uncontrollable temper; Mendeleev finished at the top of his class.

Dmitri Mendeleev (1834-1907). From Wikimedia Commons, user Materialscientist.
In one of his later works, he made this dedication: “This investigation is dedicated to the memory of a mother by her youngest offspring. Conducting a factory she could educate him only by her own work. She instructed by example, corrected with love, and in order to devote him to science she left Siberia with him, spending thus her last resources and strength.”
This education, however hard-won, prepared him to teach at the gymnasium level as his father did, a prospect he did not relish. After a brief teaching stint in Odessa (in Ukraine), Mendeleev applied to the Main Pedagogical Institute to work on his magister degree (similar to a master's degree), but the Institute had shut down, so he applied to the St. Petersburg Imperial University. He was accepted and was awarded his first and second Magister degrees in chemistry within a year. For the next two years, Mendeleev gave seminar lectures and supervised undergraduate laboratory studies, all at an inadequate salary. His work was sufficiently impressive that the university granted him a twenty-two-month fellowship to study abroad. After touring Europe for a while and meeting many influential chemists, Mendeleev settled on Heidelberg as a place to study. There he met Robert Bunsen (1811-1899), but Bunsen's lab lacked the equipment for the research Mendeleev was doing, so he bought the needed equipment and set up a lab in his apartment. In Heidelberg, Mendeleev met fellow chemists Emil Erlenmeyer (1825-1909) and Alexander Borodin (1833-1887). In addition to his career in chemistry and medicine, Borodin became famous as a symphonic and operatic composer.
Mendeleev met many other chemists at Karlsruhe Congress, possibly including Julius Lothar Meyer, but there is no specific record of their meeting. Denied any extension of his education abroad, Mendeleev returned to St. Petersburg, just as the serfs were becoming emancipated. Although he got an adjunct teaching position, societal and student unrest closed the university. He needed to do something to avoid starving, so he wrote a textbook on organic chemistry. The book was five hundred pages long and was completed within sixty-one days. The hurry was occasioned by the deadline to compete for the Demidov Prize (the Russian equivalent of the Nobel Prize) from the Academy of Sciences. The book was completed in time, and Mendeleev won the prize in 1862. The prize money enabled him to marry Feozva Nikitichna Lescheva. Although strongly encouraged by Mendeleev's sister Olga, it was an extremely unhappy union in which the two eventually were unable to tolerate the presence of each other in the same house.
Mendeleev's career prospered, however. He completed his PhD dissertation and taught at several institutions in St. Petersburg, winding up at St. Petersburg State University. With all the societal reforms, higher education became much more popular, and Mendeleev's freshman chemistry course became quite crowded. Further, there were no suitable texts, so Mendeleev set out to write a Russian-language text for inorganic chemistry. The six hundred pages of volume 1 dealt with only eight elements: hydrogen, oxygen, carbon, nitrogen, fluorine, chlorine, bromine, and iodine. Since he had the entire rest of the elements to deal with in volume 2, Mendeleev knew he had some serious organizing to do. He spent about six weeks at the start of 1869 experimenting with various arrangements of the remaining elements.
I began to look about and write down the elements with their atomic weights and typical properties, analogous elements and like atomic weights on separate cards, and this soon convinced me that the properties of elements are in periodic dependence upon their atomic weights.4
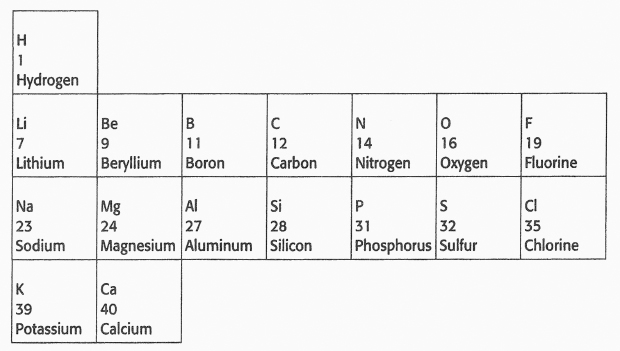
The lightest element, hydrogen, was the sole member of the first row. The second row began with lithium and continued all the way to fluorine. When Mendeleev reached the next element, sodium, an element whose properties were quite similar to those of lithium (e.g., reacting violently with water), he started a new row, placing sodium below lithium. He continued along this row until the pattern repeated with potassium (which also reacted violently with water), which he placed below sodium. Next, he placed calcium below magnesium and beryllium.
MIND THE GAP
After calcium, the next known element in order of atomic mass was titanium. If Mendeleev placed titanium immediately following calcium, it would occupy a place directly below aluminum. But Mendeleev knew from his study of the chemical properties of boron and aluminum that titanium did not fit into that group: boron and aluminum form compounds with oxygen in which the ratio of boron or aluminum atoms to oxygen atoms is two to three. Using subscripts to indicate the relative number of atoms, these compounds can be represented by the formula E2O3, where E represents an element in the boron-aluminum group. Titanium forms a compound with oxygen, whose general formula corresponds to that of the carbon-silicon group, namely, EO2. Because titanium's chemical properties more closely matched those of the elements in the carbon-silicon group than those in the boron-aluminum group, Mendeleev boldly skipped one space, placing titanium below silicon, as shown below:
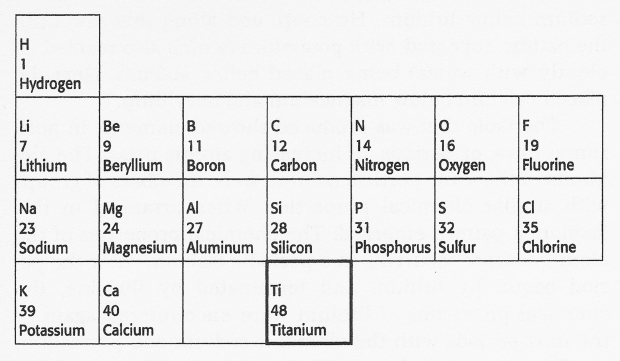
This space or gap was actually a prediction. Mendeleev's hypothesis was that the chemical properties of the elements recur in a periodic fashion or, more specifically, that the chemical properties of the elements are periodic functions of their atomic masses. Based on this hypothesis, Mendeleev predicted that another element should exist that fits the blank space in the periodic array of elements. This element should have properties similar to boron and aluminum and should have atomic mass between those of calcium (40) and titanium (48). His prediction was found to be correct when scandium (Sc), atomic mass 45, was discovered in1879.
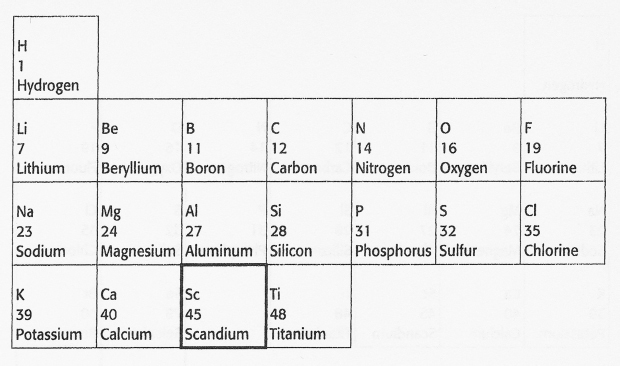
Mendeleev also predicted the existence of two other as yet undiscovered elements, which he called ekasilicon and ekaluminum. In this sense, Mendeleev's periodic table is a hypothesis, and the skipped spaces are predictions of missing elements. As we have seen, experimental evidence that matches predictions lends support to the hypothesis. Besides the discovery of scandium in 1879, the other two elements predicted, called germanium and gallium, were found in 1886 and 1875, respectively.
REENTER MEYER
Meanwhile, in Germany, Julius Lothar Meyer had been working on revisions to his table of elements, and several versions predated Mendeleev's. So, why didn't Meyer get credit? Two reasons: First, Mendeleev's publication of the complete table preceded Meyer's, a fact alluded to in Meyer's book. Second, Mendeleev made his predictions with much more specificity about the chemical and physical properties of the predicted elements. Although a few of Meyer's German colleagues pressed the case for his priority, he himself never did. One interesting event from 1882 was that Mendeleev and Meyer shared the British Royal Society's Davy Medal in 1882 “for their discovery of the periodic relations of the atomic weights.” Julius Lothar Meyer became the first chemistry professor at the University of Tübingen and taught there until his death in 1895.
SPECIAL DISPENSATION
Mendeleev wasn't quite done. In 1876, while still married to Feozva, he met seventeen-year-old Anna Ivanova Popova, his niece's best friend. This beautiful young woman became an obsession with Mendeleev, and he resolved to marry her or else jump into the ocean and drown. Unable to receive an immediate divorce through the Orthodox Church, Mendeleev nevertheless found a priest to marry him and Anna. He avoided prosecution as a bigamist by appealing to the czar. When another nobleman desirous of the same dispensation also appealed to Czar Alexander, alluding to Mendeleev, the czar is reported to have replied, “Mendeleev has two wives, yes, but I have only one Mendeleev.”5
In later years, Mendeleev's eccentric tendencies became even more evident. His piercing stare riveted his (mostly undergraduate) students, and his beard and long hair (trimmed yearly) added to his image. Mendeleev's strong democratic beliefs also flourished. He delivered a student protest to the Ministry of Education and was rewarded with an official rebuke. In 1890, he resigned his teaching post and entered government service. Mendeleev worked for the modernization of Russia in many ways, rising to the position of director of the Central Board of Weights and Measures. Does this remind you of Isaac Newton (chapter 3) who became Master of the Royal Mint?
NOBEL PRIZE CONTROVERSY
In 1905 Mendeleev was elected a member of the Royal Swedish Academy of Sciences. The following year the Nobel Committee for Chemistry recommended that the Swedish Academy award the Nobel Prize in Chemistry for 1906 to Mendeleev for his discovery of the periodic table. The Chemistry Section of the Swedish Academy supported this recommendation. The academy was then supposed to approve the committee choice as it had done in almost every case.
At the full meeting of the academy, a dissenting member of the Nobel Committee, Peter Klason, proposed an alternate candidate for the prize: Henri Moissan, who had isolated the element fluorine. Another influential academy member, Svante Arrhenius, argued that the periodic table concept was too old to win the prize in 1906. Some suggested Arrhenius still held a grudge against Mendeleev for his criticism of Arrhenius's dissociation theory, which said that acids dissolve in water to form two charged substances called ions. Remarkably, Moissan's nomination won the majority vote of the academy.
In 1907, Mendeleev died from influenza at the age of seventy-two in Saint Petersburg. The large impact crater Mendeleev on the far side of the moon, as well as element number 101, the radioactive mendelevium, are named after him. When he died, students carried the periodic table in the funeral procession.
MOSELEY'S ATOMIC NUMBERS TO THE RESCUE
Despite its usefulness, Mendeleev's periodic table was based entirely on empirical observation supported by very little understanding. It turns out that a measure unknown in Mendeleev's time, the atomic number—the number of protons in the nucleus of an atom—is a more fundamental guide to correlating chemical properties than atomic mass.

Henry Moseley (1887-1915). From Wikipedia, user Deglr6328.
The person who contributed this vital piece of information was a young British physicist named Henry Gwyn Jeffreys Moseley (1887-1915).
H. G. J. Moseley was born in the town of Weymouth, England, in 1887. He was educated in private schools and won a scholarship to Eton College, probably Britain's most prestigious prep school. At age eighteen, he won Eton's physics and chemistry prize. In 1906, he was admitted to the University of Oxford's Trinity College, where he studied physics. Then, in 1910, he moved to the University of Manchester to join Ernest Rutherford's research group.
In 1913, Moseley celebrated his twenty-sixth birthday. Mendeleev's periodic table was forty-four years old and had grown in size as new chemical elements were discovered and added to it. However, a basic flaw remained in Mendeleev's table: the position predicted by an element's atomic mass did not always match the position predicted by its chemical properties.
Moseley began to examine the question of whether elements could have a more fundamental property than atomic mass. He had learned from William and Lawrence Bragg (unique father-and-son Nobel prize-winners for X-ray studies that led to crystallographic studies that determined DNA structure; see chapter 13) that when high-energy electrons hit solids such as metals, the solids emit X-rays. He wondered if he could study these X-rays to learn more about what goes on inside atoms.
Moseley put together an experimental apparatus to shoot high-energy electrons at different chemical elements and measure the wavelength and frequencies of the resulting X-rays. He discovered that each element emits X-rays at a unique frequency. His data made most sense if the positive charge in the atomic nucleus increased by exactly one unit as each element in Mendeleev's periodic table is examined in its turn. This meant that Moseley had discovered that the basic difference between elements is the number of protons they contain.
He said, “We have here a proof that there is in the atom a fundamental quantity, which increases by regular steps as one passes from one element to the next. This quantity can only be the charge on the central positive nucleus, of the existence of which we already have definitive proof.”6
His hypothesis about the placement of each element in a periodic table was that it corresponded to its atomic number. Argon, for example, although having an atomic mass greater than that of potassium (39.9 versus 39.1, respectively), was placed before potassium in the periodic table. While analyzing the frequencies of the emitted X-rays, Moseley noticed that the atomic number of argon is 18, whereas that of potassium is 19, which indicated that they were indeed placed correctly. Moseley also noticed three gaps in his table of X-ray frequencies, so he predicted the existence of three unknown elements: rhenium, discovered in 1925; technetium, discovered in 1937; and promethium, discovered in 1945.
In its modern form, the periodic law can be stated thusly: The chemical properties of the elements are periodic functions of their atomic numbers. Tellurium, atomic number 52, thus precedes iodine, atomic number 53. In a sense, Mendeleev was lucky, for increasing atomic mass is almost always correlated with increasing atomic number.
When World War I began in 1914, Moseley enlisted as a volunteer in the British Army's Royal Engineers. His family pleaded with him to continue his scientific research, and the army was reluctant to accept him. Moseley had to fight hard to get into the army.
Second Lieutenant Henry Moseley was killed in battle by a sniper at the age of twenty-seven in Gallipoli, Turkey, on August 10, 1915. His grave is located on Turkey's Gallipoli Peninsula. As a result of Moseley's death, and after much lobbying by Ernest Rutherford, the British government placed a ban on other scientists of repute serving in wartime front-line roles.
Robert Millikan (1868-1953), winner of the Nobel Prize in Physics in 1923, wrote: “Had the European war had no other result than the snuffling out of this young life, that alone would make it one of the most hideous and most irreparable crimes in history.”7 In 1916 no Nobel Prizes were awarded in physics or chemistry. There is a strong consensus that Henry Moseley, had he been alive, would have received one of these awards. (Nobel Prize-winners must be alive at the time of presentation.)
ROOM TO GROW
The number of known elements has increased greatly since the days of the ancient Greeks. There are now 118 known elements, each of which is described quite well by the periodic law. In order to accommodate all of these elements, the periodic table has been modified in form and expanded. As a result, scandium is no longer placed under boron and aluminum, and titanium is no longer located under carbon and silicon.
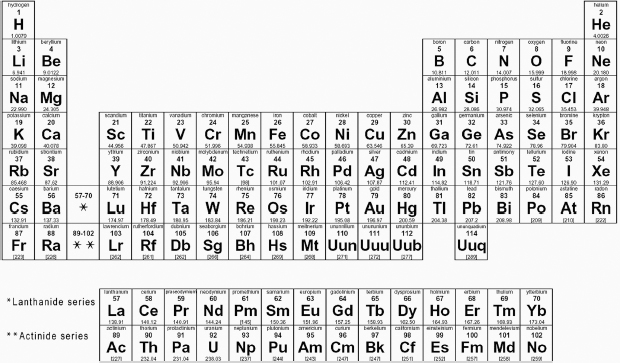
Modern periodic table. Courtesy of wpclipart.com.
THE NAME GAME
In the past, the group that first synthesized an element got the privilege of naming it. Recently, however, rules regarding the nomenclature of new elements have been enacted to prevent acrimonious conflict over who gets to name a newly synthesized element.
A case in point deals with the naming of element 105, the element containing 105 protons in its nucleus. The Soviets may have synthesized a few atoms of element 105 in 1967 at the Joint Institute for Nuclear Research in Dubna, Russia, USSR, but because the Dubna group did not propose a name for the element at the time they announced their preliminary data—a practice that has been customary following the discovery of a new element—it was surmised by American scientists that the Soviets did not have strong experimental evidence to substantiate their claims. Soviet scientists contended, however, that they did not propose a name in 1967 because they preferred to accumulate more data about the chemical and physical properties of the element before doing so. After completing further experiments, they proposed the name nielsbohrium.
In 1970, a group of investigators at the Lawrence Radiation Laboratory of the University of California at Berkeley announced that they could not duplicate the Soviet experiment but were able to produce an isotope (another form of the same element with a different number of neutrons in the nucleus) of element 105. The Americans referred to this isotope as hahnium-260 because they wanted the new element to be named in honor of Otto Hahn, the discoverer of nuclear fission (see chapter 12). (The 260 refers to the number of protons and neutrons in the nucleus of this isotope of element 105.)
In 1985, the International Union of Pure and Applied Chemistry (IUPAC) decided to set up an ad hoc working group to consider the competing claims for priority of discovery of elements 101 through 112. The group first met in Bayeux, France, in February 1988. It published its final report five years later in August 1993.
At its thirty-eighth general assembly, held in 1995 at the University of Surrey in Guildford, England, the IUPAC decided to reconsider its recommended names. Following a further two years of consultation, the union ratified a slate of names for elements 101 through 109 at its thirty-ninth general assembly in Geneva in 1997. The names met with widespread approval. Element 105 was dubbed dubnium (symbol Db) after the name of the city where the Soviet scientists worked.

Used with permission from Sidney Harris.
Where are all these conflicts coming from?
As if element conflicts aren't enough, the next chapter will deal with electrical conflicts. The excitement continues.